Abstract
Introduction: Inhibitors of signaling downstream of the B-cell receptor have a demonstrated clinical benefit in a number of lymphoid malignancies but generally require chronic therapy with the potential for single mutations to lead to resistance. GS-4059 (ONO-4059) is a Bruton's tyrosine kinase (BTK) inhibitor. GS-4059 is safe and tolerable as a single agent at doses up to 480 mg in non-Hodgkin lymphoma and up to 600 mg in chronic lymphocytic leukemia (CLL). Idelalisib, a phosphatidylinositol-3-kinase delta (PI3Kd) inhibitor, is approved for the treatment of CLL. Single-agent therapy leads to durable responses, but with limited depth of response; treatment with a combination of GS-4059 and idelalisib has the potential to lead to deeper and more durable responses at lower doses of individual agents than needed as monotherapy.
Methods:This ongoing, phase 1b study (NCT02457598) is evaluating the safety and tolerability of GS-4059 in combination with idelalisib. Patients with previously treated CLL, FL, small lymphocytic lymphoma (SLL), mantle cell lymphoma (MCL), marginal zone lymphoma (MZL), Waldenstrom's macroglobulinemia (WM), or non-germinal-center B-cell type (non-GCB) diffuse large B-cell lymphoma (DLBCL) and no prior exposure to BTK or PI3Kd inhibitors eligible for enrollment. Patients are enrolled using a 3+3 dose escalation design with a fixed dose of idelalisib (50 mg BID) and increasing doses of GS-4059. Optional dose expansion cohorts of up to 30 patients can be enrolled to generate disease-specific data. Patients were observed for a 28-day period to identify dose-limiting toxicities (DLTs). Efficacy evaluation was performed at 6-week intervals for DLBCL, 24-week intervals for CLL, and 12-week intervals for all other indications.
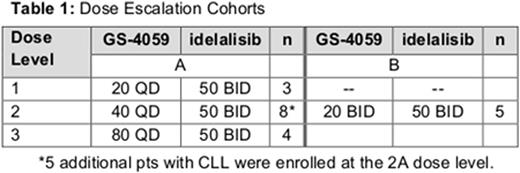
Results: As of June 1, 2016, 20 patients have enrolled; the median age was 64 (37-79) years and 65% were men. The disease subtypes enrolled were CLL (n = 8), FL (5), MZL (2), SLL (2), and 1 each with DLBCL, MCL, and WM. The median number of prior therapies is 2.5 (range 1-4). The median duration of treatment is 113 days (range 29-310) with 17 patients still on-treatment. Three patients discontinued all study treatment due to disease progression (FL, MZL, DLBCL). There has been 1 death on study following progressive disease. Two DLTs of neutropenia were observed at dose level 2B (GS-4059 20 mg BID/idelalisib 50 mg BID), prompting the decision to discontinue the evaluation of twice-daily administration of GS-4059 when combined with idelalisib. The maximum tolerated dose (MTD) was not reached in Arm A of the study (Table 1).
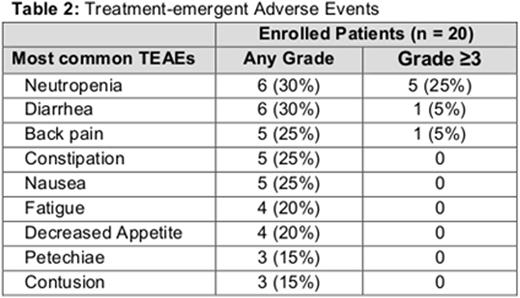
Of the 20 patients enrolled, 95% reported a treatment-emergent AE (TEAE), of which 55.0% were ≥grade 3. The only ≥grade 3 TEAE that was present in more than 1 patient was neutropenia. The most common TEAEs are listed in Table 2. Grade 3 liver laboratory test abnormalities were observed in 1 patient after approximately 5 months of treatment; a liver biopsy revealed a lymphocytic infiltrate consistent with CLL. Dose interruption due to an AE was reported in 45% of patients. Aside from 2 patients who discontinued idelalisib due to neutropenia and restarted therapy on GS-4059 alone, all patients successfully re-initiated therapy with both agents after treatment interruption. Nine patients have been on study for ≥24 weeks with 7 patients evaluable for radiographic response; 3 patients have had a >50% decrease in lymphadenopathy (CLL, SLL, FL). Preliminary pharmacokinetic (PK) results indicate that idelalisib at the evaluated dose levels does not significantly alter the PK of GS-4059.
Conclusion: Once-daily dosing of GS-4059 up to 80 mg in combination with idelalisib 50 mg BID was generally safe and well tolerated. Early results show efficacy at combination doses significantly below the MTD for either single agent. This data supports continued clinical evaluation of the combination of GS-4059 and idelalisib for the treatment of B-cell malignancies.
Salles:Mundipharma: Honoraria; Novartis: Consultancy, Honoraria; Roche/Genentech: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Gilead: Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Amgen: Consultancy, Honoraria. Morschhauser:Celgene: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria; Janssen: Honoraria; Servier: Consultancy, Honoraria. Cheson:Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Acerta: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Research Funding. Fegan:AbbVie: Honoraria; Roche: Honoraria; Gilead Sciences: Honoraria. Nelson:Gilead Sciences: Employment, Equity Ownership. Yang:Gilead Sciences: Employment, Equity Ownership. Mitra:Gilead Sciences: Employment, Equity Ownership. Starodub:Bayer: Consultancy; BMS: Speakers Bureau; Sandoz: Consultancy. Dyer:Roche: Consultancy, Speakers Bureau; ONO Pharmaceuticals: Research Funding; Gilead Sciences: Consultancy, Other: Travel funding, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal