Abstract
Background
Waldenström macroglobulinemia (WM) is a rare, indolent immunoglobulin M- associated lymphoplasmacytic lymphoma characterized by the alternation in the MYD88 locus in 90-95% of the cases. MYD88L265P is the most common alteration in WM patients and is considered a key molecular signature with a pathophysiological role in WM. Because of extremely low prevalence of WM with MYD88 wild type (WT) genotype there is a paucity of clinical and outcome data in this unique patient population. A recent study (Treon et al, Blood May, 2014), in an analysis based on the patients' MYD88 status, reported on the clinical features including older age at presentation and an unexpectedly higher mortality (38%) in the MYD88WTcohort compared to MYD88L265P patients (mortality 6%, P < .0001). However, the sample size of the MYD88WT WM cohort was small (n=15), with a few events during the short follow-up (median 4.84 years). Herein, we report on larger cohort of WM patients with this rare genotype.
Methods
This study included patients evaluated at Mayo Clinic, Rochester in whom the diagnosis of WM was established based on their clinicopathologic features and the MYD88 mutation status was determined through AS-PCR (assay sensitivity 1% mutant allele) performed on archived bone marrow samples obtained between 2007-2014. Patients with MYD88WT status were the focus of this study. Clinical data were collected from the patients' medical records. The Kaplan-Meir method was utilized for the survival analysis calculated from the time of diagnosis of WM. Outcomes of MYD88WT patients were compared with those harboring the MYD88L265Pmutation.
Results
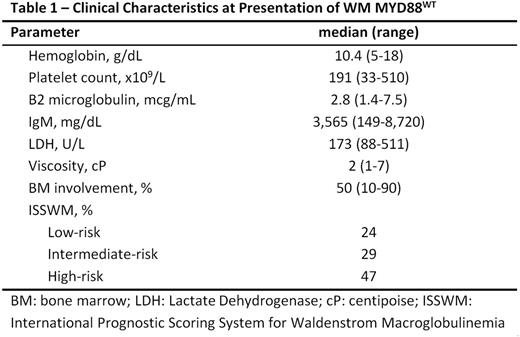
Our cohort of 171 patients with an established diagnosis of WM and known MYD88 mutation status was found to be enriched for MYD88WT (n=40) patients. All patients required therapy. At diagnosis, the median age of the patients with MYD88 WT was 63 years (range 37.5-83.5 years; comparable to that of the MYD88L265P cohort, median 65 years, range: 32-92 years, p=0.16). Familial WM was identified in 10% of patients. Sixty four percent of patients presented with constitutional symptoms at diagnosis. These symptoms included fatigue (45%), weight loss (17%), night sweats (8%) dizziness (10%), dyspnea (23%), bleeding (10%), headache (10%) and hyper viscosity syndrome (5%). Splenomegaly and lymphadenopathy was observed in 20% and 27% of patients, respectively. Thirteen percent of patients had concomitant AL amyloidosis. Additional baseline characteristics are reported in Table 1. Transformation to a higher grade lymphoma was noted in 9 (23%) of patients, four of whom had received a purine analog and /or chlorambucil previously.
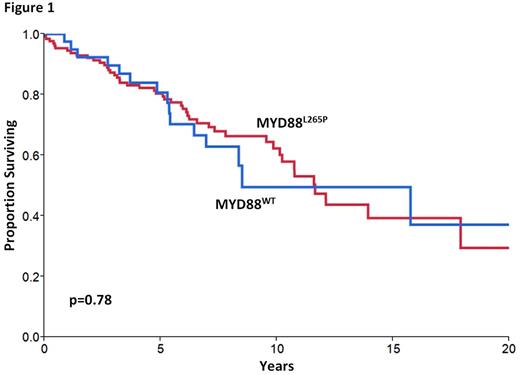
The estimated median follow of MYD88WT patients was 8.1years (95% CI: 6.4-8.9) and their median overall survival (OS) was 8.5 years (95% CI: 6.4-29.4). No OS difference was evident between this cohort and the remainder of the patients with the MYD88L265Pmutation (n=131, median OS 11.7 years, 95% CI: 10-18, p=0.7; median follow-up 7.7 years 95% CI: 6.4-9.1, Figure 1).
Conclusion
In this relatively large cohort of WM MYD88WT patients with a prolonged follow-up, the MYD88mutation status was not found to be a determinant of patients' outcomes. Our findings contradict the results of a seminal previous study and require external validation, preferably through prospective studies.
Kapoor:Amgen: Research Funding; Takeda: Research Funding; Celgene: Research Funding. Ansell:BMS, Seattle Genetics, Merck, Celldex and Affimed: Research Funding. Kumar:Sanofi: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Glycomimetics: Consultancy; Onyx: Consultancy, Research Funding; Noxxon Pharma: Consultancy, Research Funding; Array BioPharma: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Skyline: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy; Millennium: Consultancy, Research Funding; AbbVie: Research Funding; Kesios: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal