Abstract
Background:
In follicular lymphoma (FL) patients treated with first-line R-CHOP, early progression of disease (POD) within 2 years after diagnosis is associated with high risk for death (hazard ratio 6.4) and a 50% 5-year overall survival (Casulo et al. JCO 2015). Whether these observations hold for patients treated without chemotherapy is unknown. The Alliance for Clinical Trials in Oncology conducted three frontline rituximab-based non-chemotherapy-containing biologic immunotherapy doublet clinical trials: R-Galiximab (Anti-CD80, CALGB 50402), R-Epratuzumab (Anti-CD22, CALGB 50701) and R-Lenalidomide (CALGB 50803). We performed a retrospective analysis of 174 patients to determine outcomes of early progressors after initial biologic, non-cytotoxic treatment and risk factors for early POD.
Methods:
CALGB 50402 (n=60), CALGB 50701 (n=57), and CALGB 50803 (n=57) had similar eligibility criteria: previously untreated follicular lymphoma, grade 1, 2 or 3a with stage III, IV or bulky (single mass >7 cm) stage II disease, and ECOG PS 0 to 2. Early POD was defined as progression within 24 months from study entry. Univariate and multivariate logistic regression modeling using forward selection was performed to identify predictors of early POD. Kaplan-Meier (KM) method was used to estimate 2-year and 5-year overall survival probability. Hazard ratios (HR) and 95% CI were calculated using a univariate and multivariate Cox regression model adjusting for FLIPI.
Results:
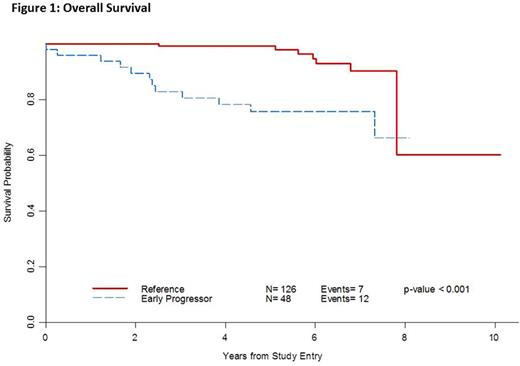
Twenty-seven percent (48/174) of patients had early POD. Median survival follow-up time from study entry was 5.5 years (2.1 to 10.1 years) and median time from diagnosis to enrollment was 2 months (0.2 to 115 months). Median age was 54 (range: 22-90), 49% were male and 24% had low-, 52% intermediate- and 24% high-risk FLIPI (Table 1). Early POD from study entry conferred a worse OS [HR=4.86 (95% CI 1.90-12.4), p < 0.001]. After adjusting for FLIPI, patients with early POD from study entry had a worse OS compared with patients who did not progress within 2 years [HR=4.77 (95% CI 1.70-13.4), p=0.003]. For early POD, the 2-year survival probability was 89% (76-95%) vs. 100% for non-early POD, and the 5-year survival was 76% (60-86%) vs. 99% (95-99%), respectively (Figure 1). When the 2-year early POD interval was taken from time of diagnosis, similar findings were noted (n=171, HR for OS 5.27 (95% CI 1.98-14.0), p 0.0009) [Table 2].
Univariate analysis revealed age >60 (p=0.019), male sex (p=0.002), higher FLIPI (p<0.001), hemoglobin <10 (p=0.021), number of nodal sites >4 (p=0.010), elevated LDH (p=0.004), nodal size >7 cm (p=0.002), albumin <3.5 (p=0.030) and CD10 positivity (p=0.015) were associated with early POD, while grade and bone marrow involvement were not. Histologic biomarkers PD1 and Ki67 were not associated with early POD in this analysis. A multivariate logistic regression model showed that male sex, albumin <3.5, low absolute monocyte count, interfollicular CD10 expression and high-risk FLIPI were predictors of early POD.
Conclusions:
Early relapse within 2 years after diagnosis in patients receiving front-line rituximab-based biologic non-cytotoxic therapy is associated with an increased risk of death. These data are similar to previous findings in patients treated with R-CHOP from the National LymphoCare study, suggesting that the adverse survival of patients with early POD may be independent of systemic treatment modality. Novel clinicopathological approaches are needed at diagnosis to identify patients who are likely to have unfavorable outcomes, and for whom biologic doublets are efficacious.
Support: U10CA180821, U10CA180882.ClinicalTrials.gov Identifier: NCT00117975 (CALGB 50402), NCT00553501 (CALGB 50701), and NCT01145495 (CALGB 50803)
Lansigan:Pharmacyclics: Consultancy; Teva: Research Funding; Celgene: Consultancy; Spectrum: Consultancy, Research Funding. Cheson:Gilead: Research Funding; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Acerta: Membership on an entity's Board of Directors or advisory committees, Research Funding. Czuczman:Celgene: Employment. Martin:Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Other: travel, accommodations, expenses; Teva: Research Funding; Acerta: Consultancy; Novartis: Consultancy; Gilead: Consultancy, Other: travel, accommodations, expenses. Hsi:Eli Lilly: Consultancy; Abbvie: Consultancy; Cellerant Therapeutics: Consultancy; Seattle Genetics: Honoraria; HTG molecular diagnostics: Honoraria. Bartlett:Gilead: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal