Abstract
Introduction: Incorporating cytogenetics into risk stratification for the treatment of childhood ALL has contributed to increased survival rates. However approximately 25% of patients, the B-other subgroup, harbour none of the known major chromosomal abnormalities. Within this group, Philadelphia-like (Ph-like) patients show a similar gene expression profile to those with the BCR-ABL1 fusion and share the same high risk of relapse. Ph-like ALL is genetically heterogeneous with some patients harbouring tyrosine kinase activating gene fusions, such as EBF1-PDGFRB, or activation of the JAK-STAT signalling pathway, making them amenable to targeted therapy with kinase inhibitors.
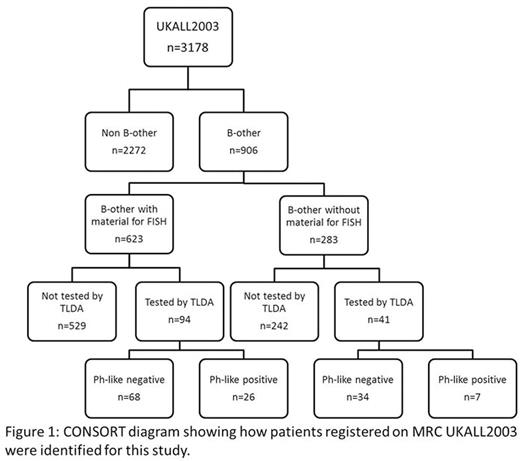
Methods: We studied patients with B-other ALL entered to the MRC UKALL2003 treatment trial using a panel of break-apart FISH probes to identify rearrangements of PDGFRB/CSF1R , ABL1 , ABL2 and JAK2. Rearrangements of the CRLF2 gene were identified using break-apart FISH probes for CRLF2, P2RY8 and IGH and/or MLPA using the P335-ALL-IKZF1 kit (MRC Holland, The Netherlands). Where possible, partner genes were identified by cytogenetics and FISH. RNA samples from 135 of the B-other cohort were screened using a Taqman low density array (TLDA) to detect the Ph-like gene expression signature and P2RY8-CRLF2 gene fusion. The overlap between the two cohorts is shown in Figure 1.
Results: Within this B-other cohort, rearrangement of CRLF2 was the most prevalent abnormality, occurring in 13% of patients (n=65/503), with two-thirds of them showing deletion of the PAR1 region, resulting in P2RY8-CRLF2 fusion (n=43) and the remaining third showing IGH-CRLF2 (n=22). Rearrangements of PDGFRB/CSF1R occurred in 3.4% of the cohort(n=17/491), while ABL1 (n=4/485), ABL2 (n=1/454) and JAK2 (n=2/481) rearrangements were rare, each occurring in less than 1% of B-other patients. Partner genes were identified in 17 cases with kinase rearrangements, with EBF1-PDGFRB being the most common (n=10). PAX5-JAK2 was identified in 2 patients and ATF7IP-PDGFRB, MEF2D-CSF1R, SSBP2-CSF1R, ETV6-ABL1 and ZC3HAV1-ABL2 in single cases.
By TDLA, 33/135 (24%) patients tested positive for a Ph-like signature. In 9 patients the arrays indicated high expression of CRLF2 with 7 of them having a P2RY8-CRLF2 transcript by TLDA, which was confirmed by FISH in 5 cases and MLPA in another case. In the remaining patients, the mechanism of CRLF2 over-expression remains unknown as no P2RY8-CRLF2 transcript was detectedby TDLA and no material was available for FISH or MLPA. A single case negative for Ph-like signature tested positive for P2RY8-CRLF2 transcript. Seven patients showed an ABL-class rearrangement by cytogenetics and FISH (EBF1-PDGFRB, n=5; ETV6-ABL1, n=1; ABL1 rearrangement partner unknown, n=1). The genetics underlying the remaining cases is being further investigated by whole genome and transcriptome sequencing. Of the 102 patients negative for a Ph-like signature, 71 of them were tested by FISH for kinase fusions. In a single case positive for EBF1-PDGFRB by FISH, expression of the EBF1-PDGFRB transcript was confirmed by RT-PCR.
The 5-year event free survival (EFS) of the entire B-other cohort was 82% with a relapse rate (RR) of 12%. Patients with rearrangements of PDGFRB/CSF1R (n=17)and those testing positive for a Ph-like expression signature (n=33) showed an inferior outcome, with EFS rates of 31% and 65% and RR of 64% and 28%, respectively (p<0.005). As we have previously demonstrated, patients with CRLF2 rearrangement showed a higher rate of relapse (23%), although it did not reach significance (p=0.129).
Conclusion: This is the largest unbiased cohort of paediatric B-other ALL screened for ABL1, ABL2, PDGFRB/CSF1R and JAK2 fusions. We demonstrate that FISH can reliably detect the full spectrum of these genetic changes, most of which are cryptic by cytogenetic analysis. These abnormalities were mutually exclusive with an overall frequency in B-other ALL of ~15%. Patients with rearrangements of PDGFRB/CSF1R and those testing positive for a Ph-like gene expression had high rates of relapse. Given the efficacy of tyrosine kinase inhibitors in treatment of these patients, testing for these abnormalities should be integrated into future clinical trials.
Mullighan:Incyte: Membership on an entity's Board of Directors or advisory committees; Amgen: Speakers Bureau; Loxo Oncology: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal