Abstract
Background : 1 to 2% of APL treated with ATRA and chemotherapy (CT) develop MDS/ AML (other than APL) during follow-up, a problematic side effect for a now highly curable disease. Characteristics of those MDS/AML (usual preleukemic phase and deletion of chromosomes 5 and/or 7, often complex) are those of alkylator induced therapy-related (t) MDS/AML, although CT used for APL treatment generally consists of anthracycline +/- AraC, with or without maintenance CT with 6-mercaptopurine (6MP) and methotrexate (MTX). On the other hand, we recently found that, in AML with NPM1 mutation that evolved to NPM1-negative MDS , somatic mutations present at the MDS phase were already present at AML diagnosis, suggesting presence of an underlying MDS (with secondary acquisition of NPM1 mutation) (Morin et al, NEngl J Med, in press) . Because cases of APL secondary to MDS (or MPN) have also been reported, we wondered whether MDS/AML occurring during the evolution of APL wereactual t-MDS/AML, or were underlying myeloid disorders which had progressed to APL.
Methods : Between 2006 and 2015, 956 patients with newly diagnosed APL were included in our APL 2006 trial and treated with ATRA plus idarubicin (Ida) and AraC induction chemotherapy, followed by 2 consolidation courses of Ida , with or without AraC, ATRA or ATO and 2-year maintenance with ATRA and CT with 6MP and MTX. 10 (1%) developed MDS or AML (without t(15 ;17) or PML-RARA rearrangement). Paired bone marrow samples collected at APL diagnosis and MDS/AML diagnosis from 9 of them were analyzed for gene mutations on genomic DNA in a selected panel of 30 genes (ASXL1, CALR, CBL, CSF3R, DNMT3A, ETV6, EZH2, FLT3-TKD, GATA2, IDH1, IDH2, JAK2, KIT, KRAS, NRAS, MPL, NPM1, PHF6, PTPN11, RIT1, RUNX1, SETBP1, SF3B1, SRSF2, STAG2, TET2, TP53, U2AF1, WT1, ZRSR2) by a next-generation sequencing (NGS) assay using the IonAmpliSeq Library Kit 2.0 (Thermo Fisher Scientific) and the Ion Proton system (Thermo Fisher Scientific). FLT3-internal tandem duplications were investigated by fragment analysis.
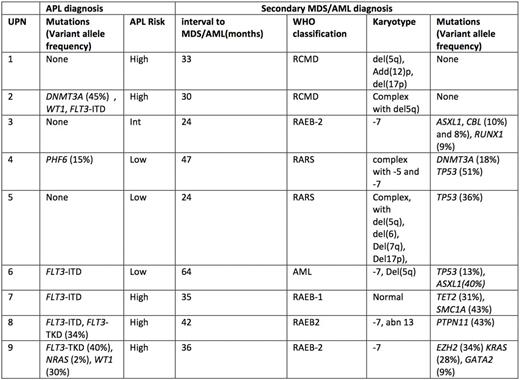
Results : Median age of the 9 cases of MDS/AML was 52 years , and median interval from diagnosis of APL treatment to MDS/AML diagnosis was 2.8 years. No MDS morphological features were reported on APL diagnosis marrow aspirates. All the patients developed MDS/AML in first CR. Karyotype at APL diagnosis found no abnormalities in addition tot(15 ;17) in the 9 pts. Karyotype at MDS/AML diagnosis showed (table) : -7/del7q (n=6), del(5q-)/-5 (n=5), del (17p) (n=2), and was complex in 5 cases.
Mutations identified at APL diagnosis (Table) were FLT3-ITD mutations (n=4), FLT3-TKD mutations (n=2), mutations in WT1 (n=2), NRAS, PHF6, DNMT3A (1 case each). At MDS/AML diagnosis, 7 patients had detectable mutations, while the remaining 2 pts had a complex karyotype typical of t-MDS/AML (table). The most frequently mutated gene at MDS/AML diagnosis was TP53 (3/9 cases), while other mutations involving ASXL1, CBL, DNMT3A, EZH2, GATA2, KRAS, PTPN11, RUNX1, TET2 ,and SMC1A were seen in one patient each, and 5 pts had several mutations. None of the mutations identified at APL diagnosis was found at MDS/AML diagnosis, and vice versa, strongly suggesting that APL and MDS/AML arose from distinct clones.
Conclusion: No MDS type mutations were found at APL diagnosis in patients who developed subsequent MDS/AML (if one excepts DNMT3a mutation, however also seen in de novo APL). Cytogenetic and mutational profiles of those MDS/AML were suggestive ofalkylator type t- MDS/AML. Thus, MDS/AML occurring during the course of APL treated with ATRA and CT have characteristics of therapy-related cases. The fact that no MDS/AML has so far been reported in large trials of APL treated with ATRA and ATO without CT also supports this hypothesis, and provides further evidence to reduce or eliminate CT from APL treatment.
Thomas:Pfizer: Consultancy. Park:Novartis: Research Funding. Ades:Celgene, Takeda, Novartis, Astex: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Fenaux:Celgene, Janssen,Novartis, Astex, Teva: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal