Abstract
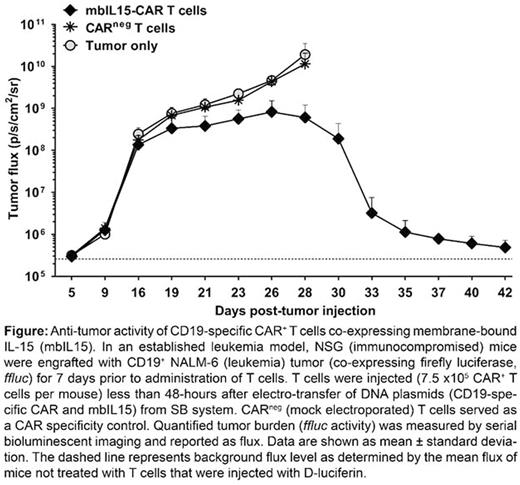
T cells are genetically modified ex vivo to express chimeric antigen receptors (CARs) for in vivo clinical applications. CAR-modified T cells have demonstrated redirected specificity and, in several clinical trials, potent anti-tumor activity. Manufacture, to date, is based upon gene transfer in cycling T cells followed by a period of tissue culture to achieve stable expression of introduced CARs. In contrast, we have adapted the non-viral-based Sleeping Beauty (SB) system to avoid the need for (i) T-cell activation and (ii) extended ex vivo tissue culture; thereby developing an approach whereby T cells can be both manufactured and delivered at multiple points-of-care (POC). This shortened culture decreases the time frame for manufacturing CAR+ T cells compared with current protocols for viral- or non-viral-based methodologies and is a foundation of our POC technology. Furthermore, reducing the ex vivo culture time preserves the memory and sustained persistence of CAR+ T cells by avoiding the differentiation programming induced by activation events typically required before or after gene transfer. We have previously demonstrated that co-expressing a membrane-bound version of interleukin-15 (mbIL15) significantly enhances the in vivo persistence of CAR+ T cells that are generated following 28-day culture after electro-transfer of SB derived DNA plasmids. Herein, we incorporated mbIL15 to generate POC CD19-specific CAR+ T cells. Peripheral blood mononuclear cells were genetically modified with mbIL15 and 2nd generation CAR coded from individual SB DNA plasmids and placed in culture for less than 2 days prior to adoptive transfer. NSG mice burdened by established and disseminated CD19+ leukemia were intravenously injected with just 7.5 x 105 CAR+ T cells, or an equivalent total T-cell dose of CARneg (unmodified or mock-treated) T cells. The mbIL15-CAR T-cell infusion yielded excellent disease-free survival, anti-tumor activity (Figure), and T-cell persistence. This approach to expediting the generation of genetically modified T cells enables the administration of CAR-modified naïve T cells and demonstrates that POC T cells have potent anti-tumor effects, even at a reduced CAR+ T-cell dose. This improvement to non-viral gene transfer and T-cell production reduces the requirement for tissue culture and thus time to manufacture within a GMP facility which translates to improvements in scalability and reduced costs. In summary, these data provide a translational pathway to undertake clinical trials by rapidly infusing T cells after genetic modification using the SB system.
Hurton:Intrexon: Equity Ownership, Patents & Royalties; Ziopharm Oncology: Equity Ownership, Patents & Royalties. Singh:Immatics: Equity Ownership, Patents & Royalties; Ziopharm Oncology: Equity Ownership, Patents & Royalties; Intrexon: Equity Ownership, Patents & Royalties. Switzer:Intrexon: Equity Ownership, Patents & Royalties; Ziopharm Oncology: Equity Ownership, Patents & Royalties. Mi:Intrexon: Equity Ownership, Patents & Royalties; Ziopharm Oncology: Equity Ownership, Patents & Royalties. Maiti:Ziopharm Oncology: Equity Ownership, Patents & Royalties; Intrexon: Equity Ownership, Patents & Royalties. Su:Ziopharm Oncology: Equity Ownership, Patents & Royalties; Intrexon: Equity Ownership, Patents & Royalties. Huls:Ziopharm Oncology: Equity Ownership, Patents & Royalties; Intrexon: Employment, Equity Ownership, Patents & Royalties. Champlin:Ziopharm Oncology: Equity Ownership, Patents & Royalties; Intrexon: Equity Ownership, Patents & Royalties. Cooper:Immatics: Equity Ownership; City of Hope: Patents & Royalties; Targazyme, Inc.: Equity Ownership; Sangamo BioSciences: Patents & Royalties; Intrexon: Equity Ownership; Ziopharm Oncology: Employment, Equity Ownership, Patents & Royalties; MD Anderson Cancer Center: Employment; Miltenyi Biotec: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal