Abstract
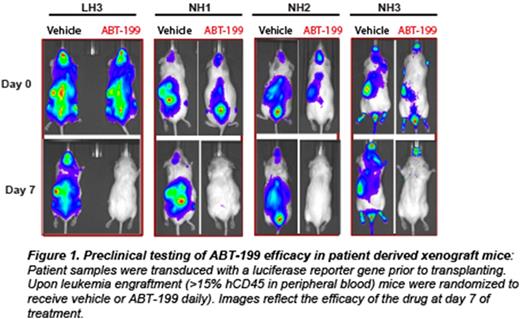
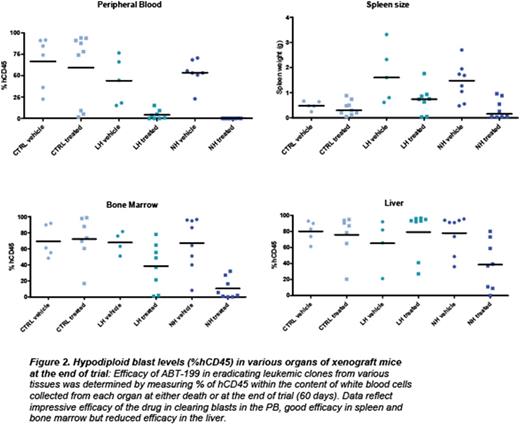
Acute lymphoblastic leukemia (ALL) is the most common cancer of childhood. Specific genetic subsets, including hypodiploid ALL, are associated with particularly high rates of relapse. Despite the poor outcomes of hypodiploid B-ALL with traditional therapeutic approaches, there have been no known effective alternative therapies or novel candidates tested to improve outcome. We hypothesized that new therapeutic targets could by identified by integrated biochemical and genomic profiling, combined with functional drug assays in order to determine which pathways play an essential role in transformation. For biochemical profiling, we analyzed multiple pathways commonly deregulated in leukemias using phosphoflowcytometry (including receptor tyrosine kinases, JAK/STAT, MAPK, PI3K, PTEN, Bcl-2 survival and pro-apoptotic family members and p53). We subjected hypodiploid cell lines (NALM-16, MHH-CALL2) and patient derived xenograft samples in vitro to inhibitors against each of these pathways (PP2:Src family;Ruxolitinib: JAK/STAT; PD235901/CI1040: MAPK; GDC-0941, PI-90, PI-103, p110 (a, b, g, d): PI3K isoform specific; PP-242:mTOR; ABT-263/ABT-737: Bcl-2/Bcl-xl, and ABT-199: Bcl-2 specific). We found that the Bcl-2 inhibitors (ABT-263, ABT-737 and ABT-199) and to a lesser extent PI3K pathway inhibitors GDC-0941 and PP-242, but not the MAPK or RTK inhibitors, efficiently reduced proliferation of hypodiploid cells. However, only ABT-263/ABT-199 induced high levels of apoptosis at nanomolar concentrations. Based on the consistent efficacy observed with ABT-199 against hypodiploid patient-derived cells and cell lines in culture, we selected eight cryopreserved, previously xenografted (F3 generation) hypodiploid patient samples (4 low hypodiploid, chromosomal number between 32 and 39; and 4 Near Haploid, chromosomal number between 24 and 31) and three non-hypodiploid patient samples (Ph-positive,Ph-Like and Erg+) for a preclinical trial in immunodeficient mice. Each patient sample was engrafted into six mice, which were randomized to receive vehicle or ABT-199 daily over 60 days (Figure 1). Treatment started when the peripheral blood (PB) human CD45 count reached 15%. A rapid decrease in PB blasts was noted at 7 days (Figure 1). Eighty-five percent of the hypodiploid xenografts survived 60 days with either undetectable or low levels of leukemia in the PB. In contrastPh+ andPh-Like xenografts died within 10-20 days regardless of treatment. Importantly, hypodiploid leukemic blasts gradually emerged after discontinuing ABT-199 after 60 days. Additionally, despite low or undetectable levels of leukemic blasts in PB and reduced levels in bone marrow and spleen, all mice had high percentages of leukemic cells in the liver (Figure 2).
In conclusion we have identified the survival protein Bcl-2 as a promising molecular target in hypodiploid B-ALL. ABT-199 for dramatically reduced leukemia cells in vitro and in vivo in patient-derived xenograft models of hypodiploid B-ALL. However, the liver represented a protective niche for these leukemias. In addition, our biochemical characterization of the organ infiltrating blasts collected from mice on trial indicate that the sensitivity of hypodiploid ALL to ABT-199 relies not only on high levels of Bcl-2 and deficiency for other survival proteins such as Bcl-xl but also on high levels of proapoptotic proteins, providing two different signatures that correlate with response to ABT-199. Using genome editing (CRISPR/Cas9) we interrogated the necessity for individual proapoptotic genes, including PUMA, NOXA, and BAD, for ABT-199-induced cell death. This study provides encouraging preclinical data that Bcl-2 may be a promising target for the treatment of hypodiploid B-ALL. Our studies identify signature biomarkers that correlate with drug response and identify essential proteins mediating ABT-199-induced cell death. Importantly, this report also identifies the limitations of using ABT-199 as single drug, and provides the rationale for using combinatorial therapies in order to improve the efficacy of the drug.
Mullighan:Loxo Oncology: Research Funding; Amgen: Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees. Loh:Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Abbvie: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal