Abstract
Background: International Working Group (IWG) has set criteria in 2003 for response assessment in patients with AML; at which time intensive chemotherapy was the main therapy (Cheson, JCO 2003). Hypomethylating agents (HMA) were approved shortly after for the treatment of elderly AML (Fenaux, JCO 2009; Dombret et al Blood 2015). Although few patients achieve a deep response, the majority will have stable disease (SD), some of which could last several months while patients continue to receive HMA and gain clinical benefit. We hereby describe outcomes for patients who achieve SD with HMA for the treatment of elderly unfit AML patients.
Methods: A total of 56 AML patients' data from Feb 2007 to June 2015 at Mayo Clinic were reviewed after appropriate IRB approval was obtained. All cases had their bone marrow slides reviewed at our institution. Patients were considered for our study if they received HMA as frontline therapy for their AML. Patients who received HMAs after induction chemotherapy failure or for their MDS were excluded. Response was identified based on 2003 IWG AML response criteria. Patients with SD were categorized into short-lasting (<6 cycles of HMA, cohort 1) or long-lasting (≥6 cycles of HMA, cohort 2) SD.Prognostic factors were analyzed by univariate and multivariate analyses. Survival estimates were calculated using Kaplan-Meier curves and univariate and multivariate analyses was based on log-rank testing using JMP software version 10.
Results:
i)Characteristics: Fifty six AML patients were treated with HMA as frontline therapy with median age of 76 years (range, 59-91), 68% of which were males. Therapy related AML (T-AML) was seen in 2% of the cases. Cytogenetics were favorable risk in 2%, intermediate in 62%, and poor in 36%. Complete remission (CR) was found in 10 (18%) patients, CR with incomplete count recovery in 3 (5%), partial remission (PR) in 3 (5%) patients. Forty patients were non-responders (SD).
ii)Characteristics of HMA SD cohorts: Upon comparison of cohort 1 versus cohort 2 there was no statistical significance in median age, white blood count, absolute neutrophil count, platelets, peripheral blood and bone marrow blasts, bone marrow cellularity and sex. Median number of cycles was expectedly higher in cohort 2 vs 1 (9 vs 2, p<0.0001). Intermediate cytogenetic risk was more common in cohort 2 vs 1 (90% vs 43%, p=0.006), while poor risk was more common in cohort 1 vs 2 (57% vs 10%, p=0.006). Only 10% had complex karyotype in cohort 2 compared to 50% in cohort 1 (p=0.017). NPM1 mutation was absent in all cases, while FLT3 mutation was present 30% in cohort 1 and 25% in cohort 2 (p = 0.9). Decitabine was more commonly given in cohort 1 vs cohort 2 (80% vs 50%, p =0.07).
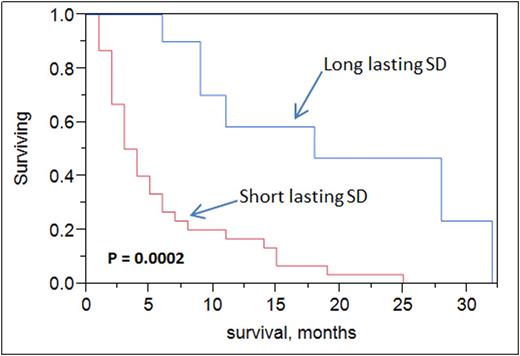
iii)Clinical outcome of HMA SD cohorts: Median overall survival was higher in long-lasting SD patients (cohort 2) vs short-lasting SD (18 vs 4 months, p = 0.0002). Upon multivariate analysis, both age (p <0.0001) and long-lasting SD (p =0.01) were statistically significant for overall survival but not cytogenetics risk grouping nor type of HMA administered.
Conclusions: Standard response criteria for AML by IWG were developed for intensive chemotherapy and do not apply to newer agents, specifically HMA. Stable disease (SD) could be meaningful to some elderly AML patients when treated with HMA. Intermediate cytogenetics and azacitidine use were more frequent in patients with long-lasting SD. Long-lasting SD did confer a survival benefit in elderly AML patients. Newer response criteria for assessing novel agents' efficacy are needed in this population.
Tibes:Italfarmaco S.p.A.: Consultancy, Honoraria. Foran:pfizer: Honoraria; Millennium Pharmaceuticals, Inc.: Research Funding; medscape: Honoraria; karyopharm: Honoraria; novartis: Honoraria; boehringer ingelheim: Research Funding; agios: Research Funding; Cellerant: Research Funding. Al-Kali:Novartis: Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal