Abstract
Introduction: Idarucizumab is a monoclonal humanized antibody fragment (Fab), designed to reverse dabigatran anticoagulation. It is approved for urgent reversal of dabigatran anticoagulant effects in massive bleeding or prior to urgent interventions. It is administered intravenously (i.v.), either as a bolus or a short infusion (2x2.5 g). In hemorrhagic shock gaining i.v. might be challenging, and alternative methods such as intraosseous (i.o.) administration are an alternative life-saving procedure. Due to the molecular size of idarucizumab (50 kDa), the efficacy and safety, including the passage from the i.o. compartment to circulation, are unknown. In this study we investigated the efficacy and the prothrombotic risk of idarucizumab, given i.v. and i.o. in a large animal polytrauma model.
Methods: After ethical approval, 21 male pigs were administered oral dabigatran etexilate for 3 days (30 mg/kg twice daily). Following anesthesia, to achieve constant plasma levels, further dabigatran was infused on day 4 (0.77 mg/kg/h for 30 min plus 0.2 mg/kg/h for 60 min). Animals were randomized 1:1:1 to receive 1) i.o. placebo (control group), 2) i.o. idarucizumab (60 mg/kg), or 3) i.v. idarucizumab (60 mg/kg). The standardized polytrauma consisted of a blunt liver injury and a femur fracture. After trauma and hemorrhagic shock, animals were resuscitated with Ringer's solution. Idarucizumab (i.o. or i.v.) or placebo (i.o.) were administered 15 min after trauma, either via an i.o. access (left tibial plateau, EZ-IO¨), or an i.v. access. Animals were observed up to 240 min after trauma, and total blood loss was assessed after the observation period or animal's death. Coagulation variables included dabigatran plasma concentration, global coagulation assays (PT, aPTT) and thromboelastometry. For a differentiated risk assessment, thrombin generation and thrombin-antithrombin (TAT) levels, indicating the extent of coagulation activation, and D-Dimers, as markers of thromboembolic risk, were obtained. Hemodynamic monitoring comprised cardiac output, mean arterial and mean pulmonary arterial pressure. For statistical analysis, ANOVA (mean ± SD) and the log-rank test for survival were used.
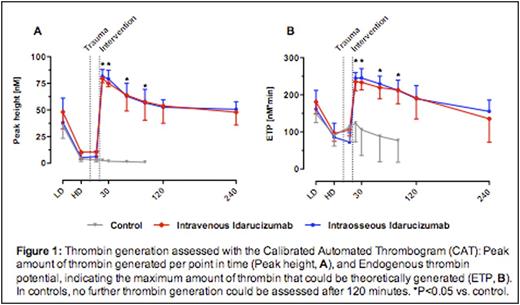
Results: Prior to trauma, all animals showed comparable plasma concentration of dabigatran (583 ± 139 ng/mL). Following idarucizumab administration, dabigatran plasma concentration was not detectable in both groups. Binding of dabigatran was associated with a normalization of whole blood and plasma coagulation variables. Total blood loss post-injury was comparable between both idarucizumab groups (i.o.: 1085 ± 102 mL, i.v.: 1142 ± 125 mL; P>0.05) with 100% survival. In contrast, control animals (no anticoagulation and no idarucizumab) exhibited the highest total blood loss, which was significantly higher than in idarucizumab treated pigs (4065 ± 557 mL, P<0.001). 6 out of 7 control animals died within the observation period (mean survival: 117 min; P<0.00001). Dabigatran concentration remained elevated throughout the observation period (last sampling point; mean: 302 ng/mL; range: 176-468 ng/mL). Thrombin generation after dabigatran infusion and trauma was not measurable. After idarucizumab, thrombin generation was restored close to baseline levels (Peak height: 128 ± 39 nM, ETP: 360 ± 106 nM*min) (both i.o. and i.v., P<0.05 vs. control). Likewise, only in idarucizumab groups TAT levels indicated re-appearance of activation of coagulation, as compared to the control group. However, there was no significant difference in D-dimers 60 min after trauma between all groups (i.o.: 1164 ± 553 ng/mL; i.v.: 1216 ± 520 ng/mL; control: 747 ± 572 ng/mL; P>0.05). Clinically no signs of thromboembolic adverse were observed.
Conclusion: The route of application (i.o. or i.v.) of idarucizumab has no impact on the efficacy of the reversal of dabigatran. Despite the size of idarucizumab, therapy can be safely applied via an i.o. access. In patients with severe bleeding and hemorrhagic shock under dabigatran application, i.o. administration of idarucizumab is a safe alternative.
Honickel:Böhringer Ingelheim Pharma GmbH & Co KG: Other: travel support. ten Cate:Böhringer Ingelheim: Consultancy, Honoraria, Research Funding; CSL Behring: Research Funding; Stago: Consultancy; Bayer: Consultancy, Honoraria, Research Funding; Philips: Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Leo Pharma: Consultancy, Honoraria. Rossaint:CSL Behring: Consultancy, Honoraria; Böhringer Ingelheim: Consultancy, Honoraria; Novo Nordisk: Consultancy, Honoraria. Grottke:Böringer Ingelheim: Consultancy, Honoraria, Research Funding; Novo Nordisk: Research Funding; Biotest: Research Funding; CSL Behring: Consultancy, Honoraria, Research Funding; Nycomed: Research Funding; Bayer: Consultancy, Honoraria; Octapharma: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Portola: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal