Abstract
INTRODUCTION: Thromboelastographic (TEG) analysis represents a global approach to monitor the clotability of the native whole blood and is sensitive to the cellular and plasma components of the blood which affect the rate or structure of a thrombus or its breakdown. This method can be used for the evaluation of the point of care hemostatic status of whole blood. rTM (recombinant Thrombomodulin) is currently developed for various clinical indications and exerts multiple actions on blood and its components to reduce thrombogenesis without increasing a bleeding risk. This is in contrast to Heparins which mediate their actions accompanied by some bleeding risk. FEIBA (Factor VII Inhibitor Bypass Activity) contains non activated Factors II, IX and X and activated factor VII. It has been approved by the FDA for use in Hemophilia A and B patients with inhibitors for routine prophylaxis and to prevent or reduce the frequency of bleeding episodes. The purpose of this study is to compare the anticoagulant effects related to bleeding risk of rTM and Unfractionated Heparin (UH) at various levels and their neutralization by FEIBA.
MATERIALS AND METHODS: Citrated Whole Blood samples were supplemented with rTM or UH in a concentration range of 0-10ug/ml. (n=10). TEG analysis was performed on a TEG 5000 system in which the clotting was initiated by recalcification of the whole blood and set parameters as R time, K Time, MA and Angle were measured. The relative neutralization profiles of the UH and rTM by FEIBA at 1 and 0.1 U/ml were also investigated. All results were analyzed in terms of Mean Values ± Standard Deviation.
RESULTS: In comparison to UH, rTM exhibited much weaker anticoagulant effects which were evident in all parametric evaluations.
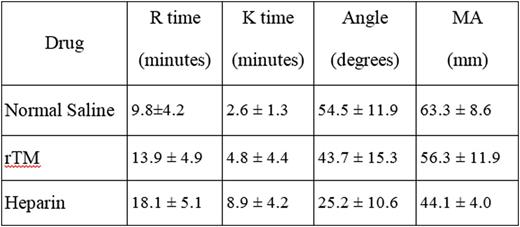
At 1ug/ml rTM did not produce any anticoagulant effects as evident by TEG profile. At higher levels greater than 2.5ug/ml, rTM produced a concentration dependent anticoagulant effect which altered the TEG profile of the whole blood. In contrast, UH produced relatively stronger anticoagulant effects and at a concentration greater than 2.5ug/ml, totally inhibited the clot formation in the TEG analysis. The anticoagulant effects of a 1ug/ml rTM as compared to 1ug/ml UH are tabulated in the following table.
At higher concentrations, UH produced strong anticoagulation of whole blood whereas rTM at a concentration of 10ug/ml produced weaker effects. FEIBA at a concentration of 1U/ml completely neutralized the anticoagulant effect of rTM at 10ug/ml and UH at 0.5ug/ml. Furthermore, FEIBA at a concentration of 0.1U/ml neutralized the anticoagulant effects of rTM completely at a concentration of 10ug/ml. At this level, it only partially neutralized the anticoagulation effects of Heparin at 0.5ug/ml. The neutralization of anticoagulation effects of rTM at 10ug/ml and UH at 0.5ug/ml by FEIBA at 0.1U/ml are tabulated in the following table.
CONCLUSION: These studies suggest that rTM is a relatively weaker anticoagulant in comparison to UH. Furthermore, in the given indications, the expected concentration of rTM is in the range of 0.5-1.5ug/ml. At these concentrations, this agent is not expected to produce any anticoagulant effects. In contrast UH, at therapeutic concentrations of 1-2ug/ml produces, strong anticoagulant effect. While these TEG studies indicate that rTM is an antithrombotic agent mediating its effects via multiple mechanisms whereas heparins primarily produce anticoagulant effects. Moreover, the supratheraputic levels of thrombomodulin and heparin are neutralizable by FEIBA.
Tsuruta:Asahi Kasei Pharma America: Employment.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal