Abstract
In acute lymphoblastic leukaemia (ALL), T cell anergy induced by tumour antigen presentation without co-stimulation contributes to immunological escape and disease progression. Cross priming of T cells by dendritic cells (DCs) may overcome this escape mechanism, restoring T cell activation and promoting a successful anti-tumour immune response. Remission induction chemotherapy induces rapid cytoreduction of leukaemia and has the modifying potential to affect effector-target cell ratios and tumour cell recognition. DCs may take up necrotic and apoptotic tumour cells, process tumour antigens, and present these to T cells alongside the costimulatory molecules required for effective priming. Further, lymphocyte recovery during remission induction chemotherapy has been linked with improved prognosis, leading to postulation that selective lymphocyte expansion may occur as an immune response against tumour cells. In this study, we extensively characterised paediatric B cell ALL patient immune cells and plasma cytokines, at diagnosis and throughout remission induction chemotherapy in peripheral blood (PB) and the bone marrow (BM) tumour microenvironment.
17 paediatric patients diagnosed with precursor B cell ALL were enrolled in this study. Matched PB and BM aspirate tissue samples were collected at time points co-ordinating with treatment protocol (diagnosis, and induction days 8 and/or 15 and 29). Mononuclear cells and plasma samples were obtained by density gradient centrifugation. DCs, monocytes, B cells, natural killer (NK) cells, T cells, invariant natural killer T cells, cytokine induced killer cells and leukaemic blasts were extensively characterised by flow cytometry in matched PB and BM aspirate samples at diagnosis, day 8, day 15 and day 29 of induction therapy. Plasma cytokines from diagnosis and day 29 samples were analysed by Luminex multi-plex immunoassay.
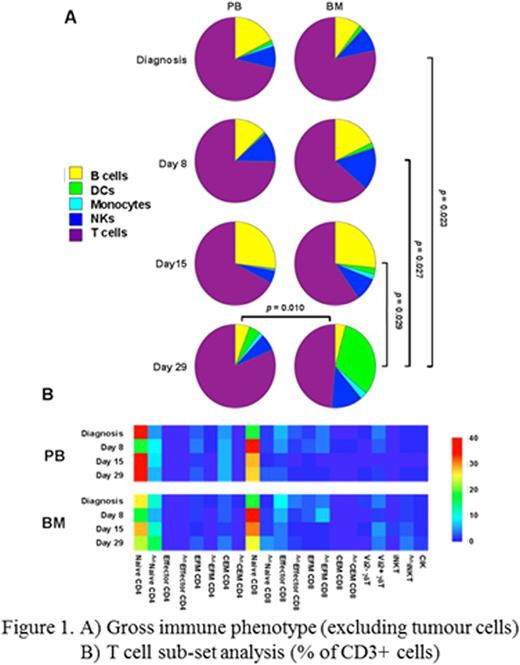
PB absolute lymphocyte counts (ALCs) at induction days 8 and 15 were lower than those recorded at diagnosis and lymphocyte recovery was observed at day 29. An equivalent pattern of lower mononuclear cell yields at days 8 and 15 with increased yields at day 29 was recorded in PB and BM samples processed for flow cytometry. All major immune cell populations were identified in PB and BM at all time points. BM DCs were significantly increased at day 29 compared to earlier time points and compared to matched day 29 PB DCs (Figure 1A). Naïve (CCR7+, CD45RA+) T cells dominated the peripheral and BM T cell pools at all time points with only low numbers of effector (CCR7-, CD45RA+), effector memory (CCR7-, CD45RA-) and central memory (CCR7+, CD45RA-) T cells recorded. However, activated (HLA-DR+) naïve CD4 T cells were significantly increased in the BM at day 29 compared to matched PB (Figure 1B) concordant to increased BM DCs. BM soluble cytokine analysis confirmed the presence of IL-1β, TNFα, IL-2, IL-4, IL-12p70 and IFNα in day 29 samples which may provide stimulus in the BM microenvironment for maturation of DCs and subsequent priming and activation of T cells.
Immunological alterations in the BM tumour microenvironment may be reflective of an altered capacity to mount an effective anti-tumour immune response. Particularly, T cell reconstitution following induction chemotherapy may have important implications in childhood leukaemia, especially if recovering T cells are able to mediate anti-leukaemia effects. Rapid lymphocyte recovery has previously been associated with improved survival, and has been shown to be accounted for by increasing T cell numbers following induction chemotherapy in paediatric ALL. Here, while ALC recovery was recorded at day 29, extensive immune phenotyping of PB samples confirmed that recovery of T cell numbers is related to an increase in naïve T cells. T cell expansion associated with a specific immune response would be expected to occur in the effector and memory T cell compartments and the increased naïve T cell numbers observed here likely relate to homeostatic proliferation or increased thymic output. However, BM DCs were increased at day 29 alongside increased activation in naïve CD4 BM T cells, which may represent initiation of an anti-leukaemia T cell response. In vitro studies to investigate patient T cell response to autologous tumour cells are required as confirmation and are currently ongoing.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal