Abstract
Sickle cell disease (SCD) is associated with chronic activation of coagulation and an increased risk of venous thromboembolism. Traditionally, it is believed that during venous thrombosis, red blood cells (RBC) are simply trapped within fibrin-rich thrombi and do not actively affect thrombosis. However, a study from our group showed that factor XIII (FXIII) activity is critical for the retention of RBC within clots and directly affects thrombus size. Others reported that during clot contraction polyhedral shaped RBC formed a densely packed core and that SCD alters the formation of polyhedrocytes which may affect clot stability (Strauss et al, 2015, ASH abstract).
We further investigated if SCD affects the structure and the dynamics of clot formation. Ex vivo clot retraction was performed using blood from sickle cell patients and Townes mice (a model of SCD). Citrated blood was added to siliconized wells of 96-well plates containing CaCl2 (10mM) and tissue factor (1pM) and incubated for 2 hours at 37°C. The number of RBC extruded from the clot was counted in serum by Hemavet™ and expressed as a percentage of initial RBC number in the anticoagulated blood. Morphology of the clots was evaluated using scanning and transmission electron microscopy (EM). Thrombosis in Townes SS (sickle) and AA (control) mice was studied using femoral vein thrombosis induced by electrolytic injury and inferior vena cava stenosis models.
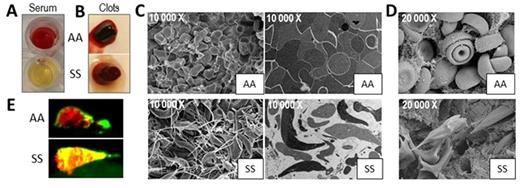
The number of mouse SS RBC in the serum extruded during clot contraction was dramatically reduced compared to the number of AA RBC (0.8±0.8% vs. 19.4±0.8%, n=3, p<0.0001, Fig. A). A similar result was observed for human RBC. Since SS mice and sickle patients have a lower hematocrit (HCT), we investigated if the number of RBC affects the extrusion of these cells during clot retraction. Indeed, lowering HCT in AA mouse blood reduced RBC extrusion from the clots. However, increasing HCT in SS mouse blood to that of AA blood did not increase the number of SS RBC extruded from the clot. Furthermore, inhibition of FXIIIa activity with T101 (10µM) increased the release of AA (by 64%, n=6, p<0.05) but not SS RBC from mouse clots. These data indicate that the entrapment of SS RBCs within the clot is not simply caused by lower RBC number, and is FXIII-independent. Mixing the platelet poor plasma (PPP) and cellular fraction of AA and SS mouse blood revealed that the entrapment of SS RBC is not mediated by PPP (AA blood recombined = 6.9±3.6%; SS blood recombined = 0.4±0.4%; AA cells/SS PPP = 6.7±6.4%; SS cells/AA PPP = 0.1±0.2%, n=3 per group).
Clots formed ex vivo from AA blood had a gel-like, soft structure, whereas SS clots were more firm and stiff. EM demonstrated that RBC within AA clots had polyhedral shapes and were tightly packed in the central part of the clot. In contrast, most of the mouse SS RBC did not have polyhedral shapes, underwent sickling and were not compacted within the clot. They also formed long "spicule-like" processes that intertwined with fibrin fibers (Fig. C). Similar results were observed in blood of sickle patients; however, the sickled RBC phenotype was less prominent. Importantly, sickling of RBC was observed in clots formed in the inferior vena cava of SS mice, 2 hours after vessel stenosis (Fig. D). We also performed a tPA challenge assay on clots formed ex vivo from human blood and showed that SS clots challenged with low tPA concentration (0.6nM) were more resistant to fibrinolysis compared to AA clots (clot lysis time, 714±6 vs. 388.3±120.7 minutes, n=6, p=0.08).
The electrolytic injury model of venous thrombosis was used to investigate the dynamics of clot formation in SS mice in vivo. Mice were infused with fluorescently labeled antibodies for fibrin (green) and platelets (red). Electrolytic injury was applied to the femoral vein; a relative intensity of fibrin and platelet accumulation was assessed by fluorescence microscopy for one hour at 10-minute intervals. SS mice have increased platelet and fibrin accumulation compared to AA mice (~ 2 fold, n=5-7, p<0.05 for 40, 50 and 60 minute time points). Interestingly, in the AA clots, platelets were mostly localized on the surface, in contrast to their widespread distribution throughout the clot in SS mice (Fig. E, yellow color).
Our data demonstrated that SCD alters the structure and dynamics of venous clot formation. Experiments investigating the consequence of these observations in mouse models of stroke and pulmonary embolism are currently ongoing.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal