Abstract
Introduction: Daratumumab (DARA) is a human monoclonal IgG1κ CD38 antibody that functions through multifaceted mechanisms of action, including CDC, ADCC, ADCP, induction of apoptosis and immunomodulatory activity (Krejcik et al, Blood 2016;128:384-394). DARA was added to standard of care (SOC) regimens in two randomized, controlled, phase 3 trials in RRMM: POLLUX (DARA+lenalidomide/dexamethasone [DRd] vs lenalidomide/dexamethasone [Rd]) and CASTOR (DARA+bortezomib/dexamethasone [DVd] vs bortezomib/dexamethasone [Vd]). The addition of DARA to these regimens resulted in significant improvements in PFS (hazard ratio for PFS: 0.37 and 0.39 for POLLUX and CASTOR, respectively) and ORR (POLLUX: 93% DRd vs 76% Rd; CASTOR: 83% DVd vs 63% Vd) (Dimopoulos MA et al, N Engl J Med 2016; in press; Palumbo A et al, N Engl J Med 2016; in press). To determine the ability of DARA to drive deep clinical responses beyond complete response (CR), MRD was assessed in both POLLUX and CASTOR using next-generation sequencing (NGS) of B cell receptor (Ig). This is the first comprehensive and prospective study of MRD to date in randomized phase 3 clinical trials of RRMM patients and investigates the ability of DARA-containing regimens to drive deep responses in this challenging population.
Methods: MRD was assessed in POLLUX (blinded to treatment group) at the time of suspected CR, and at 3 and 6 months post-suspected CR for patients who maintained this response. Similarly, in CASTOR, MRD was assessed for patients at the time of suspected CR (blinded to treatment group) and at 6 months and 12 months after first dose (at the end and 6 months after end of Vd background therapy, respectively). MRD was assessed on bone marrow aspirate samples prepared using Ficoll and evaluated by the ClonoSEQTM assay (Adaptive Biotechnologies, Seattle, WA, USA) at sensitivities of 0.01% (1 cancer cell per 10,000 nucleated cells or 10-4), 0.001% (10-5), and 0.0001% (10-6). The MRD negative rate per treatment arm was determined as the proportion of patients with negative MRD at any time point after the first dose and compared using the likelihood-ratio test. To allow for a stringent, unbiased evaluation of MRD in these studies, the entire intent-to-treat population was evaluated where patients were considered MRD positive if they had only MRD positive test result or had no MRD assessment. In POLLUX, 63% in DRd and 87% in Rd did not receive a MRD assessment, and for CASTOR, 76% in DVd and 87% in Vd were not assessed for MRD.
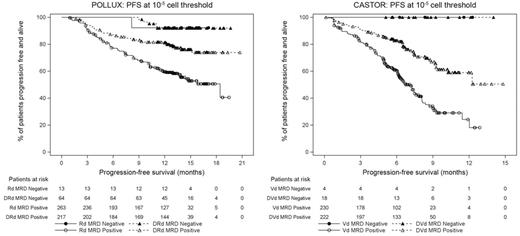
Results: Median duration of follow-up was 13.5 months and 7.4 months in POLLUX and CASTOR, respectively. The addition of DARA to SOC regimens (Rd in POLLUX or Vd in CASTOR) resulted in significantly higher MRD negative rates at all three thresholds examined (10-4, 10-5, and 10-6; Table). Additionally, patients who achieved MRD negative status experienced fewer PFS events compared with MRD positive patients at a threshold of 10-5 (Figure). Among pts who achieved CR or better, the rate of MRD negativity was at least 3-fold higher across all sensitivity thresholds in the DARA + SOC treatment groups compared with SOC alone. In MRD positive patients, PFS was significantly longer in the DARA + SOC treatment groups compared with SOC alone treatment arms. Updated data from both studies will be presented at the annual meeting.
Conclusion: These two studies represent the first randomized, controlled, prospective evaluation of MRD in the RRMM phase 3 clinical trial setting and demonstrate that DARA-containing therapies are able to drive patients to remarkably deep levels of clinical response. Regardless of the SOC component, DARA-containing regimens consistently demonstrated ≥3-fold increase in MRD negative rate compared with the control groups at all evaluated thresholds. Importantly, since patients who achieved MRD negative status demonstrated low PFS event rates, the deep clinical responses induced by DARA may lead to improved survival.
Avet-Loiseau:Celgene: Honoraria, Research Funding, Speakers Bureau; Amgen: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau; Sanofi: Honoraria, Speakers Bureau; Janssen: Honoraria, Research Funding, Speakers Bureau. Casneuf:Janssen R&D, Beerse, Belgium: Employment; Johnson & Johnson: Equity Ownership. Chiu:Janssen: Employment. Moreau:Janssen: Honoraria, Speakers Bureau; Amgen: Honoraria; Novartis: Honoraria; Takeda: Honoraria; Bristol-Myers Squibb: Honoraria; Celgene: Honoraria. Plesner:Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Khokhar:Janssen: Employment. Qi:Janssen: Employment. Schecter:Janssen: Employment, Equity Ownership. Carlton:Adaptive Biotechnologies: Employment, Equity Ownership. Qin:Janssen: Employment. Liu:Janssen: Employment; J&J: Equity Ownership; Merck: Equity Ownership. Wu:Janssen: Employment. Zhuang:Janssen: Employment. Ahmadi:Janssen: Employment. Sasser:J&J: Equity Ownership; Janssen: Employment. San-Miguel:Amgen: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Onyx: Membership on an entity's Board of Directors or advisory committees; Millennium: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; MSD: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal