Abstract
Background. It has been reported that iron absorption from gastrointestinal tract was enhanced in a subset of patients with myelodysplastic syndrome (MDS) exhibiting ineffective erythropoiesis. Iron absorption was achieved via an iron transporter ferroportin which was downregulated by hepcidin. Recently, three erythroid regulators such as growth differentiation factor 15 (GDF15), twisted gastrulation protein homolog 1 (TWSG1) and erythroferrone (ERFE) which down regulated hepatic hepcidin production has been identified. However, it has been not yet clarified which molecules could contribute to the increased iron absorption in patients with MDS.
Materials and Methods. In the present study, we examined the expression level of GDF15, TWSG1 and ERFE mRNA during ex vivo erythroid differentiation from CD34+ bone marrow (BM) cells in the presence of 4 U/mL erythropoietin (EPO), 100 U/mL interleukin-3, 10 ng/mL stem cell factor, 20 ng/mL insulin-like growth factor (IGF)-1 and 500 micro g/mL iron-saturated transferrin. We further analyzed the expression level of GDF15 and ERFE in BM mononuclear cells (MNCs) derived from BM derived from MDS patients and lymphoma patients without BM involvement as a control by using quantitive RT-PCR. The expression of EPO-R was analyzed by flow cytometry. The CD34+ MDS cells were seeded on fibronectin substratum in 5 mL of a serum-free medium supplemented with 50 ng/mL human thrombopoietin (TPO), 10 ng/mL human SCF, 50 ng/mL human Fms-related tyrosin kinase 3 ligand (FLT3LG) and 100 ng/mL human delta like protein 4 (DLL4) with or without 4 U/mL EPO. For analysis of CD34+ cells, a GEO dataset (GSE58831) was downloaded as a matrix by GEOquery package (Bioconductor). The numerical data of the matrix were normalized by quantile normalization using limma package. Clinical and sequencing data were downloaded from supplementary materials. Those were combined with a GEO dataset (GSE58831) before analysis.
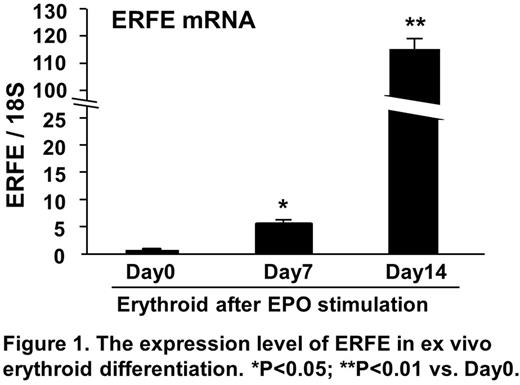
Results. The level of ERFE mRNA was dramatically increased during erythroid differentiation from control CD34+ cells in response to EPO in vitro (Figure 1) although increase of the level of GDF15 and TWSG1 was marginal. Moreover, the level of ERFE mRNA in BM MNCs derived from MDS patients was significantly higher than that from control. Furthermore, the expression level of ERFE mRNA correlated with the percentage of CD34+ cells, but not percentage of erythroblasts derived from MDS patients. Using GEO data sets (GSE58831), the level of ERFE mRNA in CD34+ cells derived from MDS patients was significantly elevated as compared with that from healthy volunteers. Importantly, flow cytometric analysis indicated that CD34+ MDS cells highly expressed EPO receptors and the level of ERFE mRNA in CD34+ cells in a subset of MDS patients was enhanced after exposure of EPO ex vivo. In addition, the level of ERFE mRNA positively correlated with the level of EPO-R in CD34+ MDS cells (GSE58831).
Conclusion. These results indicated that CD34+/EPO-R+ double positive MDS cells is one of the major sources of ERFN. The level of ERFE in CD34+ MDS cells may be associated with abnormal iron metabolism in MDS patients. These finding may be important for understanding the abnormal iron metabolism and predicting the efficacy of EPO administration.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal