Abstract
Erythroblastic islands (EBIs) are a hallmark of mammalian erythropoiesis consisting of a central macrophage surrounded by and interacting closely with maturing erythroblasts. While it is generally accepted that the island macrophages play an important role in erythropoiesis, the inability to identify and isolate this macrophage subpopulation has limited our understanding of their functional involvement. Previous studies have relied on immunohistochemistry/immunofluorescence in situ or in vitro. More recently, flow cytometry was used to characterize EBI formation and the immunophenotype of the central macrophages in murine erythroblastic islands. These approaches provide either morphological/structural information or high-throughput quantification, but not both, and often carry the expectation that all EBI macrophages have similar phenotype (F4/80+/CD169+/VCAM1+ for example), and thus potentially overlook critical information about the nature and biology of the islands and the central macrophages.
We have developed a novel method for analysis and characterization of EBI macrophages from hematopoietic tissues using multispectral imaging flow cytometry, which combines the high-throughput advantage of flow cytometry with the morphology and fluorescence details obtained from microscopy. This method allows automated, non-biased evaluation of the EBIs recovered from a sample, their number, mean size, as well as structural and morphological details of the central macrophages and associated erythroblasts. Most importantly, the images, combined with the fluorescence similarity feature, enables the evaluation of co-expression of any phenotypic markers that may be used to identify the macrophages which is crucial since some antigens used to identify macrophages (e.g. CD45, CD11b) may also be expressed on non-erythroid cells associated with the islands instead of, or in addition to, the central macrophage itself.
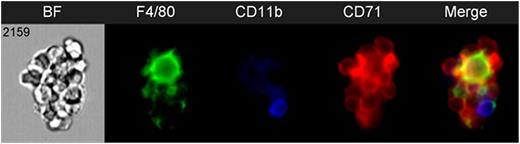
We used this method to confirm the expression of various markers previously reported to be expressed on the erythroblastic island macrophages by flow, including CD11b, VCAM1, F4/80, CD169, and CD163, in mouse, rat, and human bone marrow. Indeed, while a large number of studies have focused on murine erythropoiesis, the identity and role of the EBIs in other species is much less known. We confirmed expression of CD169 and VCAM1 on the F4/80+ central macrophages of murine EBIs and also identified a population of VCAM+/F4/80- central cells associated with developing erythroblasts. CD11b is abundantly expressed by non-erythroid, non-macrophage cells associated with the islands, but is not expressed significantly on the central macrophages (Figure 1). CD163, a marker of EBI macrophages in rat and human, was not detected in the murine EBIs by imaging flow cytometry, but this may be due to limitation of the antibodies tested. In contrast, anti-CD163 stained well rat and human EBI macrophages but CD11b or VCAM1 were not detected in EBIs from rat and human bone marrow respectively, raising the question of a species-specificity regarding the macrophage heterogeneity and satellite cells present within erythroblastic islands.
In summary, the data presented herein demonstrate the effectiveness of this method for the analysis and characterization of EBIs and establish a new tool for future investigations of EBIs and their central macrophages in the nurturing of erythropoiesis.
Representative image of an erythroblastic island harvested from murine bone marrow stained with F4/80-AF488 (green), CD11b-PE (blue), and CD71-BV421 (red) and analyzed by imaging flow cytometry.
Representative image of an erythroblastic island harvested from murine bone marrow stained with F4/80-AF488 (green), CD11b-PE (blue), and CD71-BV421 (red) and analyzed by imaging flow cytometry.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal