Abstract
Introduction: Multiple myeloma (MM) is an incurable hematologic malignancy associated with high disease burden and relapse rates. In recent years, several treatment options for MM have become available that have improved patient outcomes. However, robust data on real-world treatment outcomes associated with these MM treatments are sparse. PREAMBLE (Prospective REsearch Assessment in Multiple myeloma: an oBservationaL Evaluation; NCT01838512) is an ongoing multinational observational study that aims to increase understanding of real-world clinical effectiveness of immunomodulatory drugs (IMiDs), proteasome inhibitors (PIs), and combination therapy for relapsed/refractory MM (RRMM). Here, we present preliminary efficacy analyses on data from patients with 1 line of prior MM therapy both with and without prior transplantation experience.
Methods: Eligible patients had a confirmed diagnosis of RRMM with 1 prior treatment and started treatment with an IMiD, PI, or IMiD+PI 90 days prior/30 days following study enrollment. Patient data were collected at each healthcare provider visit for a follow-up period of 3 years. Vital status was recorded every 6 months for all patients. Response rates (defined as minimal response or better) were assessed using cumulative incidence function, with progression as competing risk. Time in response, progression-free survival (PFS), and overall survival (OS) were assessed using the Kaplan-Meier method.
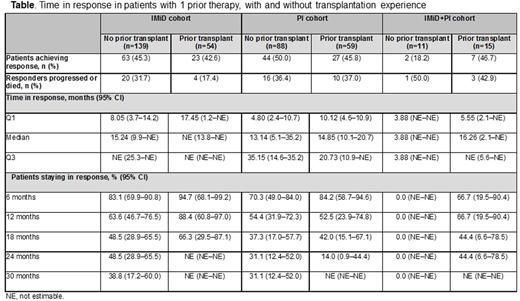
Results: Of 855 treated patients, 367 (43%) had 1 prior line of therapy (median age 70 years, 56% male). In this group, 71 (19%) had refractory disease, with even distribution among International Staging System stages I, II, and III. Index therapy was IMiD (n=193, 53%), PI (n=148, 40%), or IMiD+PI (n=26, 7%). At data cut-off (April 2016), median (Q1-Q3) follow-up was 16.7 (9-27) months, and 225 (61%) patients were still on study; the most common reasons for discontinuation were death or entering into a randomized clinical trial. Discontinuation was attributed to death for 92 (25%) patients; 69 (75%) of these deaths were due to disease progression. Approximately one-third of patients (128/367; 35%) had prior transplantation experience: 5% of patients had 2 prior transplantations, 99% of transplantations were autologous, and 83% were received after frontline (first) therapy. In patients without transplantation experience (n=238), the response rate (95% CI) was 46% (39-53) at 6 months, 58% (50-65) at 12 months, and 60% (53-67) at 18 months, versus 43% (34-53), 60% (50-70), and 60% (50-70), respectively, in those with prior transplantation. Median time in response was 14.6 months in patients without prior transplantation versus 20.3 months in those with prior transplantation. In patients with and without prior transplantation, time in response was longer in patients who had received an IMiD as index therapy (Table). Median PFS was 11.5 months in patients without transplantation and 14.1 months in those with transplantation; PFS rates (with/without prior transplantation) was: 6 months, 71%/67%; 12 months, 56%/49%; 18 months, 41%/32%. OS rate at 12 months was 81% in patients without prior transplantation and 82% in those with prior transplantation. In patients (≥6 months on study) who responded within 6 months, OS rate (IMiD/PI cohorts) was: 6 months, 100%/100%; 12 months, 93%/91%; 18 months, 85%/78%. In patients (≥6 months on study) who progressed within 6 months, OS rate (IMiD/PI cohorts) was: 6 months, 100%/100%; 12 months, 84%/69%; 18 months, 56%/64%.
Conclusions: In patients with MM and 1 line of prior therapy either with or without prior transplantation experience, approximately 45% achieved a response, with approximately 40% of these patients maintaining their response at 18 months. Regardless of index therapy type or prior transplantation experience, loss of response was observed over time, highlighting the continuing unmet medical need in RRMM. Collectively, these data exemplify the importance of novel therapies that have potential to provide durable responses and improve treatment outcomes for patients with RRMM. Further analyses exploring any impact of prior transplantation and type of frontline therapy on treatment outcomes with subsequent lines of therapy are ongoing, and will be included in the final presentation.
Study support: Bristol-Myers Squibb (BMS). Medical writing assistance was provided by K Rees, of Caudex, funded by BMS.
Durie:Janssen: Consultancy; Amgen: Consultancy; Takeda: Consultancy. Kuter:Amgen: Consultancy; Eisai: Consultancy; Genzyme: Consultancy; GlaxoSmithKline: Consultancy; ONO: Consultancy; Pfizer: Consultancy; Shionogi: Consultancy; Shire: Consultancy; 3SBios: Consultancy; Bristol-Myers Squibb: Research Funding; Protalix: Research Funding; Rigel: Research Funding. Davis:Bristol-Myers Squibb: Employment. Zyczynski:Bristol-Myers Squibb: Employment. Goldschmidt:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Chugai: Honoraria, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Millennium: Honoraria, Research Funding; Onyx: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees. Vij:Amgen: Honoraria, Research Funding; Janssen: Honoraria; Bristol-Myers Squibb: Honoraria; Celgene: Consultancy; Takeda: Honoraria, Research Funding; Novartis: Honoraria; Karyopharm: Honoraria. Popov:Bristol-Myers Squibb: Consultancy. Cella:Abbvie, Inc.: Consultancy, Research Funding; GlaxoSmithKline: Consultancy, Research Funding; Bayer Pharmaceuticals, Inc.: Consultancy, Research Funding; Alexion, Inc., Astellas, Biogen Idec, Celgene, Clovis Oncology, Inc., Daiichi Sankyo, Eli Lilly, Evidera, Inc., Exelixis, Fiborgen, Genetech, Helsinn Therapeutics, Inc., Immunogen, Ipsen Pharma, Janssen, Lexicon Pharmaceuticals, Inc., Merck, Novartis, Onc: Consultancy, Research Funding; Facit.org: Other: President; Bristol-Meyers Squibb: Consultancy, Research Funding. Cook:Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Sanofi: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Glycomimetics: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal