Abstract
Introduction: Chemotherapy induced anemia (CIA) is associated with an array of symptoms that can negatively impact patients' quality of life. The incidence and severity of CIA vary significantly depending on the cancer type and chemotherapy regimen administered. Several patient characteristics, such as age, gender, renal function and pre-treatment hemoglobin (Hb) and albumin level have also been reported to be associated with the risk of CIA. However, a comprehensive risk prediction model for CIA is lacking. Here we sought to develop a risk prediction model for severe CIA (Hb<8 g/dl) in breast cancer patients that accounts for detailed chemotherapy regimens and novel risk factors for anemia.
Methods: Women diagnosed with incident breast cancer at age 18 and older between 2000-2012 at Kaiser Permanente Southern California (KPSC)and initiated myelosuppressivechemotherapy before June 30, 2013 were included. Women who did not have any hemoglobin measurement prior or during the course of chemotherapy were excluded. Those who had the following conditions prior to chemotherapy were also excluded: less than 12 months KPSC membership, anemia, transfusion, radiation therapy or bone marrow transplant. Potential predictors considered included established CIA risk factors, such as patient demographic characteristics, cancer stage at diagnosis, chemotherapy regimens, and laboratory measurements (Table 1). In addition, several novel risk factors were also evaluated for their ability to predict severe CIA; these included recent cancer surgery and radiation therapy, chronic comorbidities (Table 1) and mediation use (Table 1).All data were collected from KPSC's electronic health records. The cohort was randomly split into a training set (50%) and a validation set (50%). Logistic regression was used to develop the risk prediction model for severe CIA. Predictors that showed a crude association with severe CIA with an odds ratio > 1.5 or <0.67 (i.e., 1/1.5) or a p-value <0.10 in the training set were included for predictive model selection. A stepwise model selection method was used with a p-value cut-off at 0.05. The model performance of the selected final model was evaluated in the validation set usingHosmer-Lemeshow goodness of fit test and the area underthe receiver operating characteristiccurve (AUC).
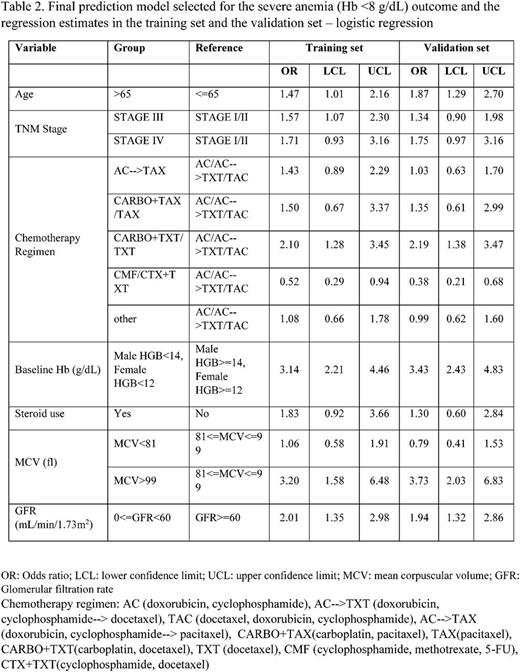
Results: A total of 11,291 breast cancer patients were included in the study. The mean age at diagnosis was 55 years. The majority of the patients were of non-Hispanic white race/ethnicity (57%). Of these, 3.0% developed severe CIA during chemotherapy. The following factors were positively associated with risk of developing severe anemia in the crude analyses and were thus included for model selection: age >65, advanced stages, length of KPSC membership, time between cancer diagnosis to chemotherapy, prior radiation therapy, vascular disease, renal disease, hypertension, osteoarthritis, use of steroids, use of diuretics, use of calcium channel blockers, use of statins, chemotherapy regimens, prior surgery, anti-coagulant use, calendar periods, and baseline ALP, HCT, HGB, lymphocyte count, MCH, MCV, ANC, platelet, RBC, RDW, WBC and GFR (calculated from creatinine). The final model included age, stage, chemotherapy regimen, corticosteroid use, and baseline Hb, MCV and GFR. The odds ratio and 95% confidence interval estimates of variables in the final model in the training set and the validation set are both shown in Table 2.
This prediction model achieved an AUC of 0.76 in the validation set, and passed the goodness-of-fit test (test statistics was 0.17).
Conclusion: The risk prediction model incorporating traditional and novel CIA risk factors appeared to perform well and may assist clinicians to increase surveillance for patients at high risk of severe CIA during chemotherapy.
Chao:Amgen Inc.: Research Funding. Xu:Amgen Inc.: Research Funding. Family:Amgen Inc.: Research Funding. Xu:Amgen Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal