Abstract
Background: More frequent molecular monitoring (qPCR tests) and higher adherence to TKIs in the management of CML have been associated with better clinical and economic outcomes. In addition, more frequent qPCR tests have been associated with better adherence to TKIs. This study estimated the overall impact of more frequent qPCR tests on healthcare resource utilization(HRU) and costs, stratified by direct (impact of qPCR test frequency on HRU and costs) and indirect (through adherence to TKIs) impacts.
Methods: Adult patients newly diagnosed with CML who started first-line therapy with a TKI (imatinib, nilotinib, or dasatinib) were identified from two US administrative claims databases (2010-2015). Adherence to TKIs (using Medication Possession Ratio), number of inpatient (IP) days, emergency room (ER) visits, and days with outpatient (OP) services were measured during the first year of CML treatment. Mean costs per event (USD 2015 - payers' perspective) were estimated for each HRU component. Direct and indirect impacts of qPCR test frequency were estimated using multivariate regression models adjusting for potential confounding factors (age, sex, region of residence, health plan type, year of CML treatment initiation, Darkow disease complexity, and Charlson comorbidity index). A model was developed to assess the direct and indirect impacts of varying qPCR test frequency on HRU and costs during the first year of CML treatment under different scenarios in clinical practice. Two scenarios are illustrated: an increase i) from 1 to 2 and ii) from 2 to 4 qPCR tests.
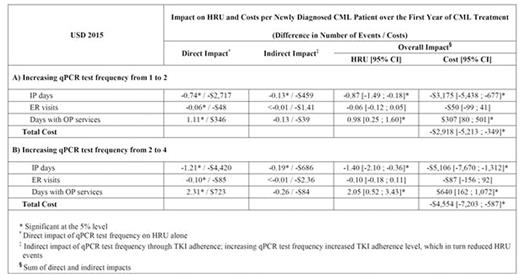
Results: A total of 1,431 patients (mean age = 54 years; 47% female) were included. During the first year of CML treatment, 36% of patients did not have any qPCR tests, 16% had 1, 15% had 2, 18% had 3, and 16% had 4 tests, for an average of 1.6 qPCR tests. The average TKI adherence level was 0.86. Holding the TKI adherence level and other variables at their mean values, the direct impact of increasing qPCR test frequency by 1 test was associated with a reduction in the number of IP days by 13.0% and ER visits by 8.3%, and an increase in the number of days with OP services by 3.0% (all p<.05). Each increase of 1 test was also associated with an increased TKI adherence level by 2.2 percentage points (p<.01). When considering the indirect impact of qPCR test frequency through adherence to TKIs, an increase of 1 qPCR test combined with an increase of the TKI adherence level by 2.2 percentage points was associated with a greater reduction of the number of IP days from 13.0% to 15.2% and ER visits from 8.3% to 8.6%, but reduced the increase in the number of days with OP services from 3.0% to 2.6%. Mean costs were estimated at $3,660 per IP day, $848 per ER visit, and $313 per day with OP services - these estimates are used for cost imputation in the Table. Thus, for a health plan of 1 million beneficiaries - considering a CML annual incidence of 1.8 per 100,000 individuals - the impact of increasing the qPCR test frequency from 1 to 2 and from 2 to 4 was associated with an estimated cost savings of $52,530 and $81,971 over a one-year period, respectively.
Conclusions: More frequent qPCR tests and better adherence to TKIs was associated with a reduction of HRU events and costs for patients and payers. Efforts to increase the qPCR test frequency and adherence to TKIs stand to enhance such benefits.
Latremouille-Viau:Analysis Group: Employment; Novartis: Other: Author is an employee of Analysis Group which has received consulting fees from Novartis. Guerin:Analysis Group, Inc: Employment; Novartis Pharmaceuticals Corporation: Consultancy, Other: Annie Guerin is an employee of Analysis Group, which has received consultation fees from Novartis Pharmaceuticals Corporation. Gagnon-Sanschagrin:Novartis: Other: Author is an employee of Analysis Group which has received consulting fees from Novartis; Analysis Group, Inc: Employment. Dea:Novartis: Other: Author is an employee of Analysis Group which has received consulting fees from Novartis; Analysis Group, Inc: Employment. Cohen:Novartis: Other: Was an employee of Novartis at the time the study was conducted. Joseph:Novartis: Employment; Amgen: Other: Stocks/stock option; Pfizer: Other: Stock/stock option.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal