Abstract
Introduction: Non-stem cell therapies including cellular vaccines and chimeric antigen receptor (CAR) T cells are experiencing explosive growth in research development and clinical applications. Demand is growing, with several CAR products heading for imminent FDA approval. The variety of products poses a challenge to even experienced cancer centers. Our goal was to create clinical and operational infrastructure to deliver these therapies efficiently and safely. We leveraged existing workflows and care models spanning inpatient and outpatient settings and disease groups previously unfamiliar with cell therapies.
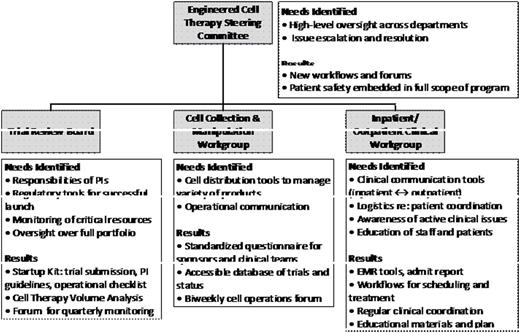
Methods: We conducted over 30 internal stakeholder interviews across research, cell processing, nursing, physician, pharmacy, and operational areas and queried experts at other cell-engineering sites. Key areas identified were 1) cell distribution, 2) trial initiation, 3) communication, training, and clinical care, 4) resource utilization, and 5) safety and outcome monitoring. A steering committee formulated guiding principles: make safety paramount, systematically coordinate care, recognize each cell therapy and sponsor's unique requirements, and minimize unnecessary infrastructure. Aspects of the care spectrum evaluated spanned from patient identification, financial clearance, outpatient workup, cell collection, admission/administration, to post-infusion follow-up.
Results: 1) Cell distribution: despite product manufacturing at central contracted sites, novel internal workflows were required to ensure chain of identity through multiple hand-offs. A standard questionnaire was devised to determine unique trial hurdles and a consistent approach to labeling, shipping and communication with sponsors and clinicians. Biweekly meetings between apheresis, cell processing, nursing, and pharmacy staff to discuss logistics and impediments were established. (Figure) 2) Trial initiation: A "start up kit" was developed to explain key entities and workflows (e.g. apheresis, pharmacy for tocilizumab, biosafety review) to help investigators unfamiliar with cell therapies navigate submission. 3) Communication, training and clinical care: guidance in budget design, tools to flag high-risk patients in electronic medical records (EMR), and education on nomenclature were developed. Over 20 general education sessions were conducted across clinical and operational areas, in addition to trial-specific training, for financial coordinators, patient safety groups, MDs, residents, PAs and nurses in all disease areas both inpatient and outpatient, even statisticians. We created email distribution lists, EMR flags, a case management system, and toxicity management order sets to ensure awareness of admissions, availability of crucial medications, recognition of cytokine release or neurotoxicity requiring unique intervention, and priming of ICU resources. A weekly forum for MDs and clinical stakeholders to share clinical events and needs was instituted. 4) Resource utilization: shortfalls were identified in cell-collection capacity and scheduling, product distribution, and clinical expertise. Dedicated clinician positions were created, and scheduling was consolidated to optimize capacity within and outside existing BMT infrastructure. 5) Safety and outcome monitoring: a format and forum was developed for quarterly review of major outcomes allowing institutional oversight over the spectrum of investigational cell therapies and standard of care administrations.
Discussion: With the explosion in outpatient cancer vaccines and CAR T cells, the need for additional physical resources, new medications, experienced clinicians, and communication tools became evident. We describe our approach to creating novel workflows to ensure patients receive the latest cell therapies safely, timely distribution of cells, educated ED and ICU involvement, and rapid expert management including anti-cytokine therapy. The investment of individual centers to bring cellular therapies safely into clinical care should not be under-appreciated, particularly given the high-risk nature of these treatments and the unique processes and high-coordinated activities these new modalities require. Regulatory agencies, such as FACT, are likewise requesting that such safety measures, standard procedures and oversight be addressed.
Soiffer:Kiadis: Membership on an entity's Board of Directors or advisory committees; Juno: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal