Abstract
Background:Over the past 2 decades, treatment advances have greatly improved the outcomes of patients with multiple myeloma (MM) and a glut of new knowledge about the biology, genetic profile, and prognostic factors of the disease have become available. However, much of this research has excluded patients with low performance status and those with impaired renal function or marrow reserve, therefore, relatively little is known about these patients who are largely ineligible for clinical trials. We performed a secondary data analysis of the Multiple Myeloma Research Foundation (MMRF) CoMMpass study to compare the disease manifestation, treatment, and prognosis of these patients with the overall MM population.
Methods: Data was extracted from the open-access MMRF Researcher Gateway corresponding with interim analysis 8 from the CoMMpass study. The CoMMpass study is enrolling 1000 newly diagnosed MM patients who will be tracked longitudinally for 5 years. CoMMpass collects relevant clinical data as well as sequential tissue samples. Eligibility requirements for CoMMpass include: symptomatic MM with measureable disease by SPEP (≥1.0g/dL), UPEP (≥200mg/24 hours), or SFLC (≥10mg/dL); receiving an immunomodulator (IMID) and/or a proteasome inhibitor (PI) for initial MM treatment; and no prior malignancies in the past 5 years.
We defined ineligibility for clinical trials as one or more of the following at time of MM diagnosis: ECOG performance status 3 or 4; creatinine ≥ 2.0mg/dL or receiving dialysis; absolute neutrophil count (ANC) <1.0x109/L or receiving growth factor support; or platelet count <50,000 x109/L or receiving platelet transfusion support. We compared the demographics, baseline presentation (International Staging System [ISS] stage, marrow and extramedullary disease burden, and chromosomal abnormalities), and first-line treatment of these patients with the overall population using bivariate analyses. We then performed a multivariate Cox regression analysis to compare event-free survival (EFS) defined as the interval from diagnosis to disease progression or death. The level of significance was set at 0.05 for all tests.
Results:799 patients were available for analysis. The median age was 64 years, 61% (486) were male, and 76% (611) were white. 23% (180) met the study definition of ineligibility for clinical trials. 5% (41/778) had low performance status, 16% (128/799) impaired renal function, 3% (22/799) impaired marrow reserve.
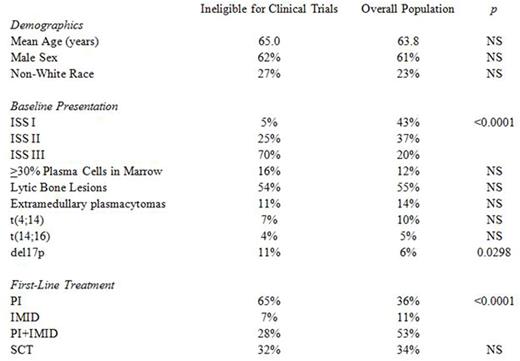
Patients ineligible for clinical trials were similar to the rest of the population demographically, but were more likely have higher risk disease by ISS (70% stage 3 compared to 20%, p <0.0001), and deletion of 17p (11% compared to 6%, p <0.0298), and were less likely to receive IMIDs during first-line therapy (35% compared to 64%, p <0.0001). Interestingly, stem cell transplant (SCT) utilization was similar between the two groups (32% compared to 34%, p >0.05). Bivariate analyses are summarized in Table 1.
After controlling for ISS stage, deletion of 17p, first-line treatment, and SCT utilization, patients ineligible for clinical trials had a 56% (aHR 1.56 [95% CI 1.09- 2.23], p = 0.0141) increase in risk of disease progression or death.
Conclusion: Newly diagnosed MM patients with low performance status, impaired renal function, or marrow reserve have long been considered a high-risk group and, thus, most clinical trials have excluded them. IMIDs are contraindicated for these patients due to its myelotoxicity which further limits treatment options. After adjusting for differences in treatment, these patients still have poorer outcomes. Additional research is needed to better understand the needs of these patients and improve their outcomes.
Vij:Karyopharma: Consultancy; Bristol-Myers Squibb: Consultancy; Takeda: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Celgene: Consultancy; Janssen: Consultancy; Novartis: Consultancy; Jazz: Consultancy; Shire: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal