Abstract
BACKGROUND: Multiple myeloma (MM) is the most common hematologic malignancy in the African American (AA) population with an incidence more than 2-3 times higher than Caucasians [Landgren O et al Blood 2006]. In the pre-novel therapy era, SEER data [1975-2008] indicated better survival outcomes for AA patients with MM. However, with the recent advent of novel drugs for treatment of MM, the survival gap for Caucasian patients with MM has closed [Ailawadhi S et al Br J Haematol 2012]. A recent pooled analysis of diagnostic cytogenetics in 292 AA MM patients [Greenberg et al Blood Cancer J 2015] reported on differences in commonly observed baseline cytogenetic abnormalities (CA) between AA and Caucasian MM patients. The large and diverse population of patients with MM at our institution prompted us to examine diagnostic cytogenetics in our MM patients along with other clinical features.
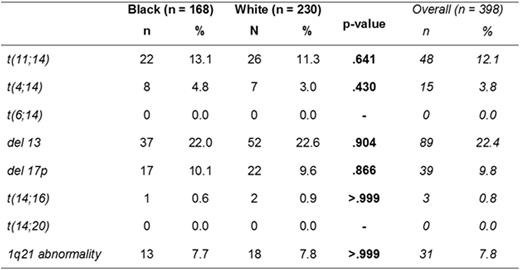
PATIENTS & METHODS: The MM database was interrogated for all patients presenting with MM between January 2012 and February 2016. Baseline clinical and pathology variables were compared between the AA and Caucasian cohorts. Continuous variables were compared using nonparametric rank tests, while incidences and proportions (e.g. CAs including t(11;14), t(4;14), t(14;16), t(14:20), amplification 1q21, monosomy13/del13q and del17p) were compared using Fisher's exact tests.
RESULTS: A total of 398 patients were identified for the analysis (African Americans n = 168, Caucasian n = 230). The median age of AA MM patients was significantly younger than Caucasian MM patients (median age 63 years vs. 68 years, p<0.0001), with a similar sex distribution. There was no significant difference in the degree of anemia, renal insufficiency, serum LDH levels, bone marrow flow cytometry, bone marrow cellularity or plasmacytosis in the two cohorts. Although there was a trend toward more ISS I amongst Caucasian MM patients, there was no statistical difference in ISS stages (p = 0.126) and no significant difference in R-ISS stage between the cohorts (p = 0.361). There was 72.7% agreement between the ISS and R-ISS staging (88 of 121 evaluable subjects had the same stage by ISS and R-ISS staging criteria), while 27.3% of the patients were upstaged from Stage I or II by ISS criteria to Stage III by R-ISS criteria. Of those upstaged, 19 patients were in the Caucasian cohort and 14 were in the AA cohort. The magnitude of this upstaging was significant when evaluated with a Generalized McNemar's test (p < 0.001). Additionally, there was a similar incidence of common FISH abnormalities in the AA cohort compared to the Caucasian cohort [Table 1].
CONCLUSIONS: This is the largest single institution report of FISH data in AA MM patients. Unlike previous reports, we show similar clinical, pathological, and cytogenetic features between AA and Caucasian patients with MM at presentation. It is possible that molecular abnormalities not detectable by FISH in our patient cohort could account for differences in our data and the published literature.
Bhutani:Prothena: Research Funding; Takeda Oncology: Research Funding, Speakers Bureau; Bristol-Myers Squibb: Speakers Bureau; Onyx, an Amgen subsidiary: Speakers Bureau. Symanowski:Eli Lilly & Co: Consultancy; Ra Pharma: Consultancy; Caris Life Sciences: Consultancy; Endocyte: Consultancy. Avalos:Seattle Genetics: Membership on an entity's Board of Directors or advisory committees. Usmani:Onyx: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Skyline: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Britsol-Myers Squibb: Consultancy, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Millenium: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Array: Research Funding; Pharmacyclics: Research Funding; BioPharma: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Research Funding, Speakers Bureau; Novartis: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal