Abstract
Background
Primary immune thrombocytopenia (ITP), an orphan disease with an estimated prevalence of 1.6 to 3.9/100,000/year, lasting for more than 12 months, is considered to be chronic ITP (cITP). The aim of treatment is to elevate platelet count and thereby reduce the risk of bleeding, whilst minimising treatment-related side effects. Following first line treatment with corticosteroids or immunoglobulins there is no clearly defined treatment pathway.
Eltrombopag a thrombopoietin receptor agonist (TPO-RA) licensed for the treatment of cITP in second line. Some patients, receive rituximab in second line; however, there is limited comparative evidence of eltrombopag and rituximab.
Aims
The aim of this Markov model is to compare eltrombopag and rituximab in terms of response rates, bleed related events and mean time to treatment failure, using a Markov model to simulate hypothetical patients' prognosis with treatments over one year time horizon.
Methods
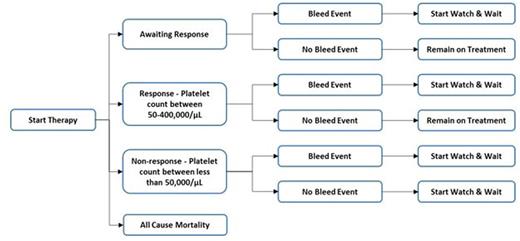
A Markov model was developed to simulate patient outcomes over a one year time horizon. The Markov model assesses response status every 4 weeks. Within each 4 weeks patients follow a decision tree (Figure 1) to determine individual treatment paths. The arrows in the diagram show the possible patient transitions. Using a Markov model allowed flexibility to accurately simulate individual treatment paths and simulate over a longer time horizon i.e., over life time.
Being in the non-response health state was associated with an increased risk of having a bleed, receiving rescue medication. Patients experiencing a bleed are at an increased mortality risk.
Treatments included in the model are associated with a response rate, time to response and treatment failure. Response was defined as per the primary endpoint from the RAISE Phase 3 trial, having a platelet level of 50-400,000/μL at assessments during treatment which were conducted weekly for the first 6 weeks and then at least once every 4 weeks thereafter. Time to treatment failure for eltrombopag was based on observed treatment duration from RAISE and EXTEND; patients who withdrew from the trial due to inadequate response or intolerant to the treatments, were considered as treatment failures. A longer time to treatment failure would denote that fewer patients experienced treatment failure and responded to treatment for longer.
Model inputs were taken from the RAISE trial for eltrombopag and from a systematic literature review for rituximab. After treatment failure, patients received watch and wait therapy which consists of IVIg, IV steroids and platelet transfusions.
Results
When simulating results for a one year time horizon, compared with rituximab, eltrombopag is associated with increased response rate, and reduced bleed related events and fewer treatment failure events.
Patients treated with eltrombopag had a 68.4% chance of being in a response state compared to treatment with rituximab which had a 38.1% chance of being in a response state. When considering a splenectomised population, eltrombopag patients had a 57.1% chance of being in a response state compared to treatment with rituximab which had a 36.3% chance of being in a response state.
Treatment with eltrombopag had a mean time to treatment failure of 7.7 months and 6.8 months for a non-splenectomised and splenectomised population respectively, a 45% increase & 38% increase compared to 5.3 month and 5.0 months for rituximab respectively for the same populations.
These translate into a 45% reduction in bleed related events for eltrombopag in non-splenectomised patients and a 31% reduction in bleed related events for splenectomised patients, compared with bleed related events associated with rituximab.
Conclusions
Eltrombopag offers improvements in response rates, increases time on response, and reduces bleed related events compared to rituximab. Eltrombopag has fewer events of treatment failures over a 1 year time horizon.
Model Schematic Repeated Every 4 Weeks
Hirst:WG Access: Consultancy. Wang-Silvanto:WG Access: Consultancy. Shephard:WG Access: Consultancy. Roy:Novartis: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal