Abstract
Background
Combination therapy has proved to be a successful strategy and generally benefits from the crosstalk of antileukemic agents. However, the mechanisms of amplification remain mostly elusive and the outcome of relapsed or chemoresistant ALL and AML is still dismal. Therefore, drug discovery is not only challenged to address the identification of novel compounds and targets, but also to enhance the understanding of their molecular interaction pathways in order to exploit their potential for the translation into combination therapies.
Screening small molecules that sensitize cancer cells towards Etoposide (VP-16) induced apoptosis, we recently introduced PS89 which targets protein disulfide isomerase (PDI) (Eirich et al, Angew Chem, 2014), a crucial enzyme for the maintenance of ER homeostasis and exciting novel target in cancer research (Xu et al, Drug Discov Today, 2014). Its chemosensitizing profile predestined PS89 for combination therapy and moreover called for the elucidation of critical networks integrated in the synergistic pro-apoptotic signaling.
Methods
Apoptosis of PS89 in combination with standard cytostatics (Daunorubicin/DNR, Etoposide/VP-16, Vincristine/VCR) was analyzed by flow cytometry in different leukemia cell lines and patient-derived xenograft (PDX) ALL and AML cells (Vick et al, PLoS One, 2014; Terziyska et al, PLoS One, 2012) versus lymphocytes of healthy individuals (at least n=3 biological replicates each). Activity-based protein profiling (ABPP) was applied in leukemic cells to identify the network of PS89 target proteins. Following interaction studies (STRING v10), biological validation of proposed communication pathways and time-dependent analysis of critical stress triggers was performed.
Results
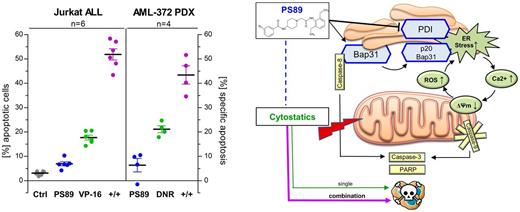
The apoptosis rate of cytostatics was synergistically enhanced in several cell line models of acute leukemia, notably applying PS89 at subtoxic concentrations in all assays (Jurkat 0.5 µM VP-16 17.7% vs PS89+VP-16 51.8%; HL-60 10 nM DNR 14.4% vs PS89+DNR 43.3%; CCRF-CEM 1 nM VCR 12.8% vs PS89+VCR 46.6%). This approach was confirmed as a therapeutic advancement in the treatment of resistant cells (VCR-resistant CEM 0.5 µM VCR 3.8% vs PS89+VCR 36.1%) as well as Pre-B-ALL and T-ALL PDX cells (n=2 diagnosis samples, 5 nM VCR 14.2% vs PS89+VCR 43.6%) or AML PDX cells (n=4 relapse samples, 20 nM DNR 38.3% vs PS89+DNR 53.6%). While in vivo studies with PDX cells are ongoing, apoptotic effects of PS89 combinations were significantly lower in isolated lymphocytes of healthy individuals which are in line with the good in vivo tolerability of PS89 (30 mg/kg i. p. daily for 2 weeks).
In a proteomics screen by ABPP in Jurkat cells, we identified the B-cell receptor-associated protein 31 (Bap31) as a further target of PS89 transducing apoptotic signals from the ER to mitochondria under ER stress (Namba et al, Cell Rep, 2013) and serving as a platform to activate caspase-8 (CASP8) following apoptotic stimuli (Iwasawa et al, EMBO J, 2011). In this regard, we proposed a distinct mechanistic pathway via the Bap31/CASP8 axis mediating the crosstalk of PDI inhibition and signaling of cytostatics.
Indeed, apoptosis induced by PS89/VP-16 combination treatment in Jurkat cells was critically dependent on CASP8 activity (4.4-fold reduction with CASP8 inhibitor Z-IETD-FMK) and co-immunoprecipitation revealed that CASP8 cleavage at the Bap31 protein complex was only detectable in the presence of PS89. In turn, active CASP8 is able to cleave Bap31 (shown in PS89/VP-16 treated cells, Western Blot) leading to ER calcium release. We observed moreover, that loss of mitochondrial membrane integrity mediated concurrently by ER calcium and p53-dependent signaling was accompanied by elevation of reactive oxygen species (ROS) levels which provokes further ER stress and finally closes the feedback loop.
Conclusion
The strong antileukemic effects of the PDI inhibitor PS89 in combination with cytostatics are orchestrated by its direct target Bap31 which transduces apoptosis signals at the ER-mitochondrial interface and emphasizes the crucial role of this social network of cell death for synergistic drug combinations. Exploiting to tune these mutual amplification loops makes PS89 uniquely applicable at subtoxic concentrations and therefore a valuable novel option to sensitize acute leukemia cells towards established chemotherapeutics.
Braig:Dr Pfleger Foundation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal