Abstract
Background:
High-dose chemotherapy and autologous stem cell transplantation is the standard treatment for relapsed aggressive B-cell non-Hodgkin's lymphomas (NHL). Carmustine, cytarabine, etoposide, and melphalan (BEAM) +/- rituximab is the preferred regimen in United States. However, many of lymphoma patients are older and unfit to tolerate full-dose BEAM. Limited observational reports suggested that modified BEAM with reduced dosages might be potential alternative. This is the first and largest subgroup analysis from a randomized phase 2 study to examine the feasibility and efficacy of reduced-dose BEAM for elderly patients.
Methods:
A total of 93 patients with relapsed aggressive NHL (53 with relapsed de novo DLBCL) were enrolled in the study period between 03/2005 and 04/2009. The initial randomization by study design was to examine the role of high-dose rituximab (1000 mg/m2) compared to standard-dose rituximab (375 m2) in combination with BEAM; we have reported no statistically significant difference in outcomes [Srour et al. J Clin Oncol 34, 2016 (suppl; abstr 7524)]. Per study design, all patients who were at age 65 years or older received reduced-dose BEAM and patients younger than age 65 received standard-dose BEAM (schedule and dosages are provided in Table 1). Primary endpoint: disease-free survival (DFS), defined as time from SCT to disease progression or death. Overall survival (OS) and safety analysis were secondary endpoints. Kaplan-Meier estimator was used to calculate survival estimates, and Cox proportional hazards regression for multivariate analysis. An intent-to-treat analysis was used.
Results
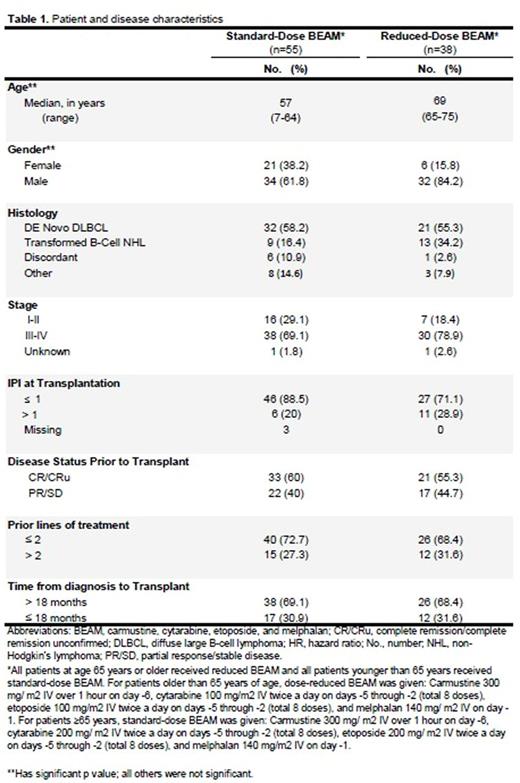
Patient and disease characteristics are provided in Table 1. Median age for patients received standard-dose BEAM (n=55) was 57 years compared to 69 years for patients received reduced -dose BEAM (n=38). With a median follow-up of 7.9 years, the 5-year DFS and OS for all patients were 40% and 48%, respectively. We found no statistically significant differences between reduced BEAM and full-dose BEAM in 5-year DFS (32% vs 46%; p=0.113). The 5-year OS for reduced-dose BEAM (age≥65 years) was 42% compared to 53% for standard-dose BEAM (p=0.039). When adjusted to other confounding factors, there were no statistically significant differences between reduced-dose BEAM and standard-dose BEAM in terms of DFS and OS. Disease status before SCT and number of prior lines of treatment were the only significant predictors for outcomes in multivariate analysis. Patients who were in remission before SCT had better DFS (HR 0.6, 95% CI: 0.36-0.97; p=0.039) and OS (HR 0.58, 95% CI: 0.35-0.98; p=0.043) than those with residual disease. Similarly, patients who had ≤2 lines of prior treatment had better DFS (HR 0.5, 95% CI: 0.3-0.84; p=0.009) and OS (HR 0.43, 95% CI: 0.25-0.73; p=0.002) than those who received >2 lines of treatments. There were no significant differences in engraftment and treatment-related mortality rates between the two groups.
Conclusion
Reduced-dose BEAM is effective, well tolerated and can be a desirable alternative for older patients with relapsed aggressive NHL. Larger prospective randomized clinical studies are of an unmet need to further evaluate the definite role of reduced-dose BEAM compared to full-dose BEAM and ASCT in this high-risk susceptible population.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal