Abstract

Hematopoietic stem cell transplantation (HSCT) associated thrombotic microangiopathy (TA-TMA) is a frequent and prognostic important complication of HSCT and may affect 20-30% of recipients. One third of these patients suffer from severe TA-TMA with a devastating prognosis. TA-TMA is a systemic small vessel disease affecting predominantly the kidney and the CNS but can affect any other organ. There is currently no accepted standard therapy for TA-TMA. The most commonly used therapeutic approaches such as plasma exchange and defibrotide are ineffective in severe TA-TMA with mortality rates of >80%. Recent data have suggested that TA-TMA, like most other forms of atypical Haemolytic-Uraemic Syndrome (aHUS) is caused by systemic activation of the alternative complement pathway. Given the high success rate of treatment of aHUS with the anti-C5 monoclonal antibody eculizumab (EC), this suggests that EC might be an effective treatment of TA-TMA. Initial reports in pediatric transplant patients seem to support this hypothesis, but the experience in adult patients is very limited and contradictory.
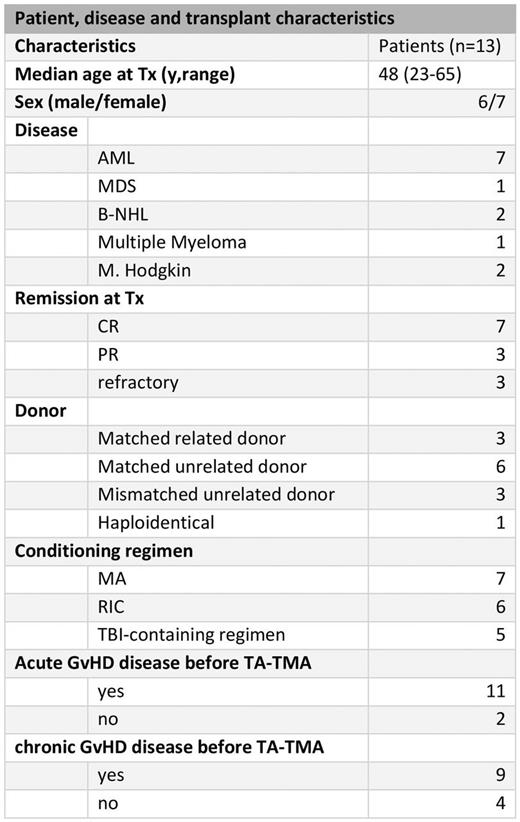
Materials and Methods: We performed a retrospective analysis of 13 patients (table 1) treated with EC in our center. Inclusion criteria were diagnosis of TA-TMA according to the Choi-criteria, significant organ damage, evidence of systemic or local complement activation by measurement of serum sC5-9 levels or tissue biopsy. The patients were treated according to the approved dosing schedule of a weekly application of 900mg EC for 4-6 weeks followed by a maintenance phase with 1200mg biweekly. EC therapy was guided by monitoring CH50 activity and serum sC5-9 concentrations as proposed by Jodele et al 2015. The EC dosing schedule was thus adjusted to maintain CH50 activity below 10% and a normal sC5-9 concentration. Clinical response was defined as improvement of organ function (e.g. no neurological residues; termination of kidney dialysis) and independence of red blood cell and platelet transfusions. All patients received ciprofloxacin prophylaxis and the majority antifungal prophylaxis with posaconazole.
Results: All patients were in remission of their underlying disease at the time of TA-TMA diagnosis a median of 9 months (from 2 to 29) after HSCT. At diagnosis 12 patients were receiving either tacrolimus (n=6), cyclosporine A (n=1) or sirolimus (n=5), which was withdrawn promptly. The median C5b-C9 level was 461 U/ml (127-810). Most patients presented with multiple organ involvement, combined CNS and kidney affection was the most common (n=8). In 9 patients EC was the primary therapy, in the other 4 patients EC was initiated after treatment failure of plasma exchange and defibrotide. The median time from TA-TMA diagnosis until EC initiation was 10 days (1-16). All patients showed sufficient blockade of the terminal complement pathway after the second EC application (CH50 <10%). A median of 9 (1-23) EC infusions were given. Clinical response was seen in 11 patients (85%) and correlated with a successful and durable normalization of sC5-9 levels. However, 9 patients (70%) died during EC therapy (4 during induction, 5 during maintenance phase) with a median survival of 3.7 months (CI95% 1.2-6.7). The main causes of death were infections (n=7) with 3 cases of aspergillosis. Progressive TA-TMA caused death in 2 patients. Multiple organ involvement (p=0.003) and active GvHD (p=0.005) at diagnosis were identified as adverse prognostic factors.
Conclusion: In our analysis we are able to show that EC shows a high response rate in severe TA-TMA patients. However, the prognosis remains very poor due to the high rate of infection related mortality which may be partially related to EC therapy. Since most patients presented with advanced, severe TA-TMA, we hypothesize that earlier diagnosis and treatment of TA-TMA and more effective prevention and treatment of infections will improve the outcome of patients with this complication.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal