Abstract
BACKGROUND
Allogeneic hematopoietic cell transplant (allo-HCT) can achieve prolonged disease free survival (DFS) in patients with high risk relapsed CLL through a graft-versus-leukemia effect. This is likely mediated through alloreactive donor T-cells that recognize and eliminate residual CLL targets expressing unique and potentially tumor-specific antigens within the context of the Class I Major Histocompatibility Complex (MHC). Histocompatibility Leukocyte Antigen (HLA) alleles encoding class I MHC can be protective or predisposing to the development of a variety of hematologic malignancies. Specifically, HLA-A*02:01, which is enriched in Caucasians, increases the risk of development of CLL, while HLA A1:01 is protective against the development of CLL. Previous single center data of reduced intensity conditioning (RIC) allo-HCT with post-transplant rituximab and donor lymphocyte infusion identified an improvement in complete remission (CR) rates and DFS in patients positive for HLA-A1, negative for HLA-A2 and HLA-B44 (HLA-A1, non-A2 and non-B44) compared to patients without these HLA alleles (Khouri, et al 2011). The impact of this combination of HLA alleles (HLA-A1, non-A2 and non-B44) on outcomes of allo-HCT for CLL has not been previously explored in a multi-center study. We therefore sought to compare outcomes of CLL patients undergoing fully-matched related or unrelated donor allo-HCT in CIBMTR centers, based on recipient HLA type.
METHODS
CLL patients ≥ 18 years old who received a fully matched allo-HCT (from matched sibling donor or 8 out of 8 matched unrelated donors) using myeloablative (MA), non-myeloablative (NMA), or reduced intensity conditioning (RIC) between 2000 and 2013 were included. Patients with Richter's transformation were excluded. Patient outcomes were compared based on the presence of the HLA combination HLA-A1, non-A2 and non-B44 (n=83) vs. other (n=481). Patient-related variables included age, gender, coexistent disease or organ impairment, HCT comorbidity score, Karnofsky score, donor/recipient CMV status. Disease-related variables included presence/absence of 17p-, 11q-, 13q-, and trisomy 12, IGHV mutation status, Rai stage at diagnosis, elevated LDH or bulky adenopathy at the time of HCT, type and number of treatments, response to pre-transplant chemotherapy, and disease status at transplant. Transplant-related variables included time from diagnosis to HCT, preparative regimen: MA vs. RIC vs. NMA, graft source, donor age, and donor-recipient sex match. Cox proportional hazard analysis was used to identify significant risk factors. For power calculation, it was assumed that overall survival (OS) at 1 year, 2 years and 3 years were 70%, 57%, and 50%, respectively. Median censoring time was assumed to be 70 months. There was 80% power to detect hazard ratio (HR) of 1.65 at the significance level 0.05. This HR was based on prior publication by Khoury et al.
RESULTS
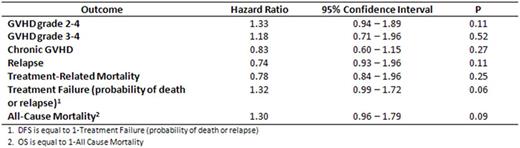
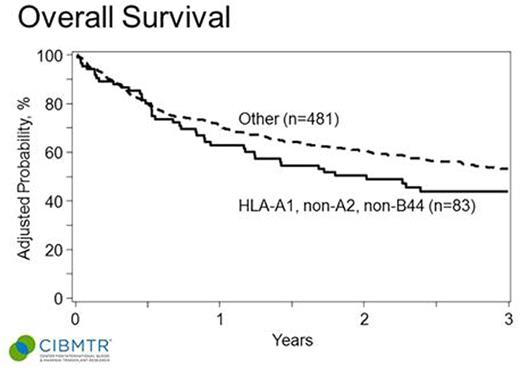
Median age of patients was 56 (range 22-75). 73% were male. Baseline characteristics between the groups with and without A1, non-A2, non-B44 were well matched in terms of prior therapy, remission status at time of allo-HCT, use of RIC or NMA conditioning, and baseline cytogenetics. In multivariable analysis, the HLA combination HLA-A1, non-A2, non-B44 was not associated with an improvement in GVHD, relapse rates, or OS (see Table ). Kaplan Meier estimates of the OS of the groups are shown in the accompanying Figure. There was a non-statistically significant trend towards improvement in DFS in patients without the HLA combination of interest (P = 0.06). In univariable analysis HLA-A1, A2 and B44 were not individually associated with OS.
CONCLUSIONS
Previously-identified HLA alleles were not associated with any measurable outcomes of allogeneic transplant from fully matched related or unrelated donors captured in the CIBMTR registry, either alone or in combination. Future study of the impact of HLA on transplant outcomes may require assessment of killer immunoglobulin receptor (KIR) subtypes for more robust exploration of the immunologic basis for differences in allo-HCT outcomes for CLL.
Hazard ratios of GVHD, relapse, TRM, Treatment Failure, and All-Cause Mortality for HLA-A1, non-A2, non-B44 vs. other
Hazard ratios of GVHD, relapse, TRM, Treatment Failure, and All-Cause Mortality for HLA-A1, non-A2, non-B44 vs. other
OS of CLL patients after HLA-matched allo-HCT based on recipient HLA type (HLA-A1, non-A2 and non-B44 vs. other).
OS of CLL patients after HLA-matched allo-HCT based on recipient HLA type (HLA-A1, non-A2 and non-B44 vs. other).
Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal