Abstract
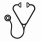
Introduction
CTL019 is a novel, investigational, chimeric antigen receptor (CAR) immunotherapy whereby autologous T cells are genetically modified with a chimeric antigen receptor to target CD19 on the surface of malignant as well as healthy B cells. The cellular kinetics of CTL019 have been evaluated in several trials for patients with relapsed/refractory CD19+ leukemias, including pediatric acute lymphoblastic leukemia (pALL), adult ALL (aALL), and chronic lymphocytic leukemia (CLL) (Maude 2014, Porter 2015).
Methods
The cellular kinetic profile of CTL019 was determined in peripheral blood (PB) and bone marrow (BM) through serial measurements using flow cytometry and quantitative real-time polymerase-chain-reaction (qPCR) assay in 3 studies comprised of (i) 55 pALL patients (NCT01626495), (ii) 28 adult CLL patients from a dose selection study (NCT01747486), and (iii) 14 CLL and 6 adult ALL patients (NCT01029366). The flow cytometry assay used a CAR19-specific anti-idiotype antibody to enumerate CTL019 T cells as a % of CD3+ T cell (Porter 2015). Cellular kinetic parameters included: maximal extent of expansion as measured by peak copies of CTL019 DNA and peak % by flow cytometry (Cmax), area under the curve at day 28 (AUC0-28d) describing expansion and persistence in the first month, and time to reach Cmax (Tmax). Parameters were derived by non-compartmental methods. Where estimable, persistence was described by the half-life (T1/2) based on the slope of the terminal phase.
Results
Following infusion, CTL019, expansion and persistence was evident in the patients who responded to CTL019 as measured by both PK assays across all 3 studies. Table 1 summarizes (arithmetic mean (SD)) the CTL019 kinetic parameters. With complete remission (CR/CRi), CTL019 cells undergo rapid in vivo expansion beyond the original CTL019 dose with maximal expansion at a mean of 11 days in pALL and aALL and approximately 14-18 days in adult CLL as determined by qPCR and flow cytometry (Table 1). In CR/CRi patients the transgene level-profiles in PB reveal a kinetic profile with an initial rapid expansion followed by a slower decay function with some fluctuations of transgene over time resulting in higher AUC0-28d and Cmax, while non-responder (NR) patients tend to have a lower expansion and faster decay (shorter T1/2)of CAR positive T-cells resulting in lower AUC0-28d and Cmax by, leaving the mechanism to be further explored. In pALL, significantly higher AUC0-28d and Cmax were observed in CR/CRi patients compared to NR patients by flow cytometry; however, a wide range of mean AUC0-28d and Cmax was observed in NR patients (n=3) resulting from significant expansion in one NR patient as determined by qPCR. In CLL, the exposure metrics AUC0-28d and Cmax were approximately 12 times higher in CR/CRi patients compared with PRi/NR/PD in NCT01747486; a similar trend was observed in NCT01029366. Similar findings were captured by the flow cytometry based measurements as summarized in Table 1. In pALL and CLL, CR/CRi patients tend to maintain higher levels of CTL019 transgene over longer periods of time (>6 months) compared to NR patients as demonstrated by the longer T1/2 value. Cellular kinetic parameters were not summarized by response category for aALL due to the small sample size (n=5 CR/CRi; n=1 NR). CTL019 transgene levels ranged from below the limit of quantification (BLQ) to 178,000 copies/ug in aALL patients with CR/CRi and BLQ to 21,900 copies/ug in the NR. CTL019 positive cells were also shown to traffic to BM at 1 month in responders (CR/CRi), irrespective of the disease.
Conclusions
Overall, significantly higher levels of in vivo proliferation and persistence were observed in patients who successfully responded to CTL019 (i.e. CR/CRi/PR) compared to NRs in both CLL and (adult and pediatric) ALL patients, as captured by both analytical measures, indicating that the kinetics of CTL019 T cells and that proliferation and persistence of CTL019 reasonably predicts response to therapy. These are the first three studies to demonstrate that cellular kinetics may predict responses to CAR based cellular therapy. These results imply that measures to increase proliferation and persistence of CAR T cells may enhance responses in resistant patients.
CTL019 concentration-time profiles for %CD3+/CTL019+ measured by flow cytometry and cellular kinetic parameters for qPCR and flow cytometry for p-ALL and adult CLL
CTL019 concentration-time profiles for %CD3+/CTL019+ measured by flow cytometry and cellular kinetic parameters for qPCR and flow cytometry for p-ALL and adult CLL
Mueller:Novartis Pharmaceuticals: Employment. Chakraborty:Novartis Pharmaceuticals: Employment, Equity Ownership. Wood:Novartis Pharmaceuticals: Employment, Other: Stock. Awasthi:Novartis Pharmaceuticals: Employment. Quintas-Cardama:Novartis Pharmaceuticals: Employment, Equity Ownership. Han:Novartis Pharmaceuticals: Employment, Equity Ownership. Maude:Novartis: Consultancy. Grupp:Jazz Pharmaceuticals: Consultancy; Pfizer: Consultancy; Novartis: Consultancy, Research Funding. Porter:Novartis: Patents & Royalties, Research Funding; Genentech: Employment. Frey:Novartis: Research Funding; Amgen: Consultancy. Marcucci:Novartis: Research Funding. Levine:GE Healthcare Bio-Sciences: Consultancy; Novartis: Patents & Royalties, Research Funding. Melenhorst:Novartis: Research Funding. June:Celldex: Consultancy, Equity Ownership; Immune Design: Consultancy, Equity Ownership; Pfizer: Honoraria; Novartis: Honoraria, Patents & Royalties: Immunology, Research Funding; University of Pennsylvania: Patents & Royalties; Tmunity: Equity Ownership, Other: Founder, stockholder ; Johnson & Johnson: Research Funding. Lacey:Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal