Abstract
Introduction
Patients with residual disease following initial treatment of chronic lymphocyticleukemia(CLL) withfludarabine, cyclophosphamide and rituximab (FCR) chemotherapy have reduced progression free (PFS) and overall survival (OS). The CLL6 RESIDUUM trial is a joint trial of the Australasian Leukaemia and Lymphoma Group (ALLG) and the French CLL branch of the French InnovativeLeukemiaOrganization (FILO) thatanalyzesthe role oflenalidomide(LEN) as consolidation therapy in patients following front-line treatment for CLL who do not enter minimal residual disease (MRD) negative complete remission.
Methods
CLL patients with CIRS score <6 requiring treatment according to iwCLLcriteria receive 6 cycles of FCR. Following completion of treatment, those with clinical, radiological and/or multiparameterflow cytometry (MFC) evidence of residual CLL in blood or bone marrow are randomized 1:1 to receive 2 years of maintenance treatment with LEN 10 mg daily or observation (OBS). Patients are reviewed for evidence of clinical progression, and peripheral blood and bone marrow are sampled regularly for evidence of MRD. CT scans are performed until resolution of lymphadenopathy and splenomegaly. Flow analysis for MRD is performed at two central laboratories using ERIC accredited methodology to achieve a sensitivity of 10-4. The primary end point of the study is time to progression or death.
Results
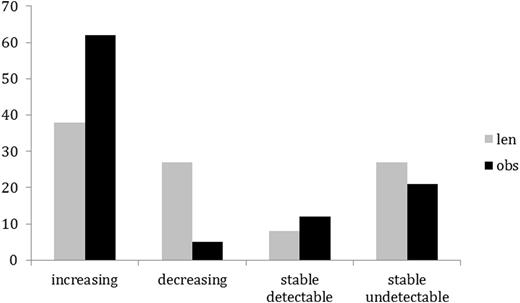
As of end of July 2016, data from 79 patients randomized on the study were analyzedfor the effects of consolidation treatment on blood and marrow MRD. Median duration from randomization was 488 days. There were 63 males and 16 females. Median age was 62 years (range 29 to 81). 37 patients were randomized to receive LEN, 42 to OBS .On the LEN arm 13, 3 and 21 patientsvs 12, 9 and 21 on the OBS arm were in CR, nodular PR and PR respectively at the time of randomization. There were 26 serious adverse events (SAEs) reported in 22 patients. 12 SAEs in 11 patients were attributed to LEN including pneumonia/chest infection (n=4), pulmonary infiltrate (1), prostatitis (1) second primary malignancy (SPM) (1), vomiting (1), neutropenia (1), tumorflare (1), acute kidney injury (1) and anal warts (1). There were 5 SAEs in the OBS arm comprising SPM (2), neutropenia (1), gout (1) and GuillainBarre syndrome(1). Peripheral blood samples were analyzedprior to consolidation and at 3, 6 and 12 months and every 6 months thereafter. MRD levels during consolidation were compared with pre-consolidation levels and categorized as increasing, decreasing, stable detectable or stable undetectable (Fig 1). MRD increased over the period of observation in 38% of patient on the LEN arm and in 62% of patients on the OBS arm (p = 0.032, Χ2). 10 patients (27%) in the LEN arm and 2 patients (5%) in the control arm had decreasing levels of MRD in the blood (p = 0.006, Χ2). There was no difference between consolidation treatments in the percentage of patients with stable blood MRD measurements, whether in the detectable or the undetectable range. The effect of LEN was most apparent in patients in PR at randomization where 5 patients (24%) taking LEN had increasing MRD in the blood compared to 15 patients (71%) on the OBS arm (p = 0.002, Χ2). Bone marrow MRD levels were assessed prior to consolidation and after 12 months in 9 patients in each arm of the study (LEN arm 2 CR, 1 nPR, 6 PR; OBS arm 4 CR, 1 nPR, 4 PR at randomization). Eight patients in the LEN arm and 2 patients in the OBS arm were observed to have a reduction in marrow MRD. There was a significant reduction between the 2 time points in the LEN arm (p=0.022 paired Wilcoxon test) but not in the OBS arm. Four of 9 patients in the LEN arm and 1 patient in the OBS arm achieved marrow MRD values below 10-4 after 1 year on trial.
Conclusion
LEN consolidation therapy for residual disease after FCR front-line therapy for CLL is associated with improved control of MRD in both blood and bone marrow. A large group of recently randomized patients will provide more data to determine whether these encouraging results will translate into improved progression free and overall survival.
Percentage of patients on LEN and OBS arms with increasing, decreasing, stable detectable or stable undetectable MRD.
Percentage of patients on LEN and OBS arms with increasing, decreasing, stable detectable or stable undetectable MRD.
Gottlieb:Indee: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees. Aurran:Janssen: Honoraria. Tam:Gilead: Honoraria; Roche: Honoraria; Abbvie: Honoraria; Janssen: Honoraria. Letestu:Roche: Honoraria; Alexion: Honoraria. Levy:Roche: Honoraria; Gilead: Honoraria; Abbive: Honoraria; Janssen: Honoraria. Leblond:Roche: Honoraria; Gilead: Honoraria; Janssen: Honoraria; Abbvie: Honoraria. Mulligan:GSK: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria, Research Funding, Speakers Bureau. Cymbalista:Abbvie: Honoraria; Roche: Honoraria; Janssen: Consultancy, Research Funding; Gilead: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal