Abstract
Introduction
The BCL-2 inhibitor venetoclax has yielded promising results in patients with relapsed/refractory chronic lymphocytic leukemia (CLL), both as monotherapy and in combination with rituximab. The CLL14 trial is a prospective, open-label, multicenter randomized phase III trial to compare the efficacy and safety of obinutuzumab and venetoclax with obinutuzumab and chlorambucil in patients with previously untreated CLL and coexisting medical conditions. Prior to opening the randomized phase, a run-in phase was performed to assess the tolerability of obinutuzumab and venetoclax in this particular patient population. Here, we report the final results on safety and efficacy of this run-in phase.
Method
The protocol specified to enroll 12 previously untreated patients with confirmed CLL and with coexisting medical conditions assessed by cumulative illness rating scale (CIRS) total score > 6 and/or estimated creatinine clearance (CrCl) < 70 mL/min requiring treatment according to iwCLL criteria into the run-in phase. All patients received 6 cycles of obinutuzumab and venetoclax followed by 6 additional cycles of venetoclax. Obinutuzumab was administered intravenously with 100 mg on day 1, 900 mg on day 2 (option to deliver 1000 mg on day 1), 1000 mg on day 8 and day 15 of cycle 1 and 1000 mg on day1 for cycles 2-6. A gradual weekly dose ramp-up of venetoclax with 20 mg, 50 mg, 100 mg, 200 mg up to 400 mg was administered starting at day 22 of cycle 1. Risk assessment for tumor lysis syndrome (TLS) based on absolute lymphocyte count and tumor burden was performed before treatment in order to direct prophylactic measures. Study defined stopping criteria for all 12 patients included: one treatment-related death or one grade 4 adverse event related to a clinical tumor lysis syndrome (TLS) despite protocol-specified prophylaxis. Adverse events were graded per the NCI CTCAE v.4 criteria. Final response to treatment including assessment for minimal residual disease (MRD) in peripheral blood by ASO-PCR was assessed per the iwCLL guidelines 3 months after the end of treatment, at month 15.
Results
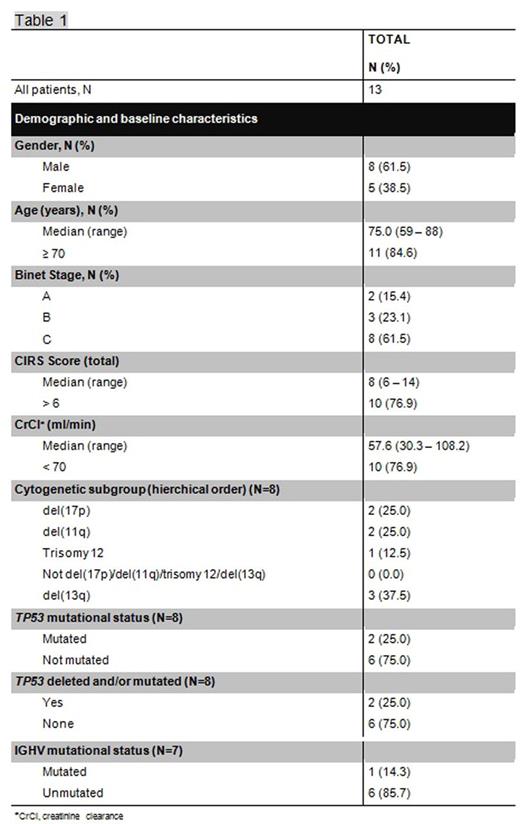
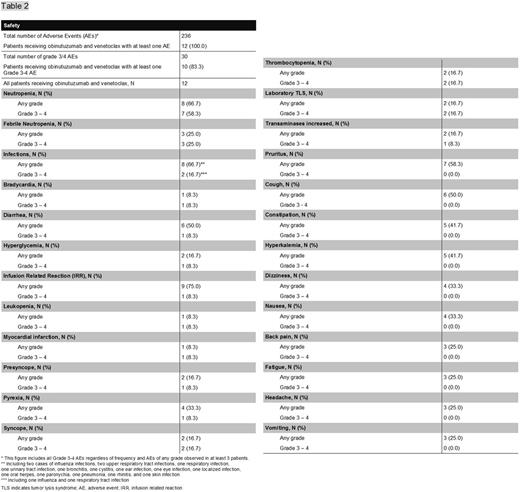
Between December 2014 and April 2015, 13 previously untreated patients from Australia, Canada, Germany, New Zealand, United States and Spain were enrolled into the trial. Baseline patient characteristics are summarized in Table 1. The median age was 75 years (range 59 - 88) and 62% of the patients were classified as Binet stage C; 38% of the patients were assessed at medium risk and 62% at high risk for TLS. One patient developed a grade-4 infusion related reaction (IRR) during the first dose of obinutuzumab and was therefore withdrawn from the trial according to the protocol requirements. Eleven of 12 patients completed treatment. One patient discontinued treatment after 6 cycles of obinutuzumab and venetoclax and 2 additional cycles of venetoclax due to patient´s wish. All patients experienced at least one adverse event. The commonest adverse events are summarized in Table 2. No clinical TLS was reported. At month 15, 11 of 12 patients were evaluable for final response assessment. All patients responded to therapy. Complete remissions occurred in 7 of the 12 patients including one complete remission with incomplete bone marrow recovery. Ten of 12 patients had no detectable (<10-4) minimal residual disease (MRD) in peripheral blood and one patient was assessed intermediate (≥10-4<10-2). At month 15, there were no events of disease progression or deaths, translating into an estimated progression-free survival of of 100%.
Conclusions
The treatment regimen developed for the experimental arm of the CLL14 trial comprising obinutuzumab monotherapy for one cycle followed by venetoclax and obinutuzumab in previously untreated, elderly patients with CLL and coexisting medical conditions appears well tolerated and effective. The target population in this trial consists of elderly patients with clinically meaningful comorbidities in addition to CLL. None of the protocol defined stopping safety criteria for the run-in phase of the trial were met. The treatment induced substantial responses with an unprecedentedly high number of MRD negative responses seen in all but one evaluable patients including those with poor prognostic features. The main phase of the CLL14 trial was opened in August 2015 and completed recruitment in August 2016 after 432 patients had been randomized.
Fischer:Hoffmann-LaRoche: Other: Travel grants. Al-Sawaf:Gilead: Other: Travel grants. Fink:AbbVie: Other: Travel grants; Mundipharma: Other: Travel grants; Celgene: Other: Travel grants, Research Funding; Hoffmann-LaRoche: Other: Travel grants. Dixon:Roche Products Limited: Employment, Equity Ownership. Bahlo:F. Hoffman-La Roche: Honoraria, Other: Travel grant. Warburton:Roche UK: Employment. Kipps:Gilead: Consultancy, Honoraria, Speakers Bureau; Roche: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Pharmacyclics, LLC, an AbbVie Company: Consultancy, Honoraria. Weinkove:Avalia Immunotherapies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Other: Travel grants; Janssen: Honoraria; Capital & Coast District Health Board: Employment; Malaghan Institute of Medical Research: Employment; Health Research Council of New Zealand: Research Funding; Australasian Leukaemia & Lymphoma Group: Membership on an entity's Board of Directors or advisory committees. Robinson:Roche: Consultancy, Honoraria, Research Funding, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; Abbvie: Consultancy; Lundbeck: Consultancy; Gilead: Consultancy. Dreyling:Roche: Consultancy, Honoraria, Research Funding, Speakers Bureau. Opat:Roche: Consultancy, Honoraria, Other: Provision of subsidised drugs, Research Funding. Owen:Pharmacyclics: Research Funding; Janssen: Honoraria; Gilead: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Roche: Honoraria, Research Funding; Abbvie: Honoraria; Lundbeck: Honoraria, Research Funding; Novartis: Honoraria. López:Novartis: Consultancy; Abbvie: Consultancy; Merck Sharp & Dohme: Consultancy; Janssen: Consultancy; Roche: Consultancy; Gilead: Consultancy. Humphrey:Genentech, Inc.: Employment. Humerickhouse:AbbVie: Employment. Tausch:Amgen: Other: Travel support; Gilead: Other: Travel support, Speakers Bureau; Celgene: Other: Travel support. Eichhorst:Abbvie: Consultancy; Mundipharma: Consultancy, Research Funding, Speakers Bureau; Roche: Consultancy, Research Funding, Speakers Bureau. Wendtner:Genetech: Consultancy, Honoraria, Research Funding; Hoffmann‐La Roche: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Munipharma: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; GlaxoSmithKline: Consultancy, Honoraria, Research Funding; Janssen‐Cilag: Consultancy, Honoraria, Research Funding; Servier: Consultancy, Honoraria, Research Funding. Langerak:F. Hofmann-LaRoche, Genentech: Research Funding; InVivoScribe Technologies: Patents & Royalties: Royalties are provided to European Network (EuroClonality). Ritgen:Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding. Boettcher:Roche: Research Funding; Abbvie: Research Funding; Celgene: Research Funding. Stilgenbauer:Mundipharma: Consultancy, Honoraria, Other: Travel grants , Research Funding; Novartis: Consultancy, Honoraria, Other: Travel grants , Research Funding; Sanofi: Consultancy, Honoraria, Other: Travel grants , Research Funding; Boehringer Ingelheim: Consultancy, Honoraria, Other: Travel grants , Research Funding; Janssen: Consultancy, Honoraria, Other: Travel grants , Research Funding; Pharmacyclics: Consultancy, Honoraria, Other: Travel grants , Research Funding; Celgene: Consultancy, Honoraria, Other: Travel grants , Research Funding; GSK: Consultancy, Honoraria, Other: Travel grants , Research Funding; Amgen: Consultancy, Honoraria, Other: Travel grants, Research Funding; Genentech: Consultancy, Honoraria, Other: Travel grants , Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel grants, Research Funding; Gilead: Consultancy, Honoraria, Other: Travel grants , Research Funding; Genzyme: Consultancy, Honoraria, Other: Travel grants , Research Funding; Hoffmann-La Roche: Consultancy, Honoraria, Other: Travel grants , Research Funding. Goede:Roche: Consultancy, Honoraria, Other: Travel grant, Research Funding; GlaxoSmithKline: Honoraria; Gilead: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Mobasher:Genentech, Inc.: Employment. Hallek:Janssen-Cilag: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; AbbVie: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; Mundipharma: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; F. Hoffmann-LaRoche: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; Gilead: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal