Abstract
Background: Novel, targeted therapies, such as ibrutinib, have transformed outcomes for patients with relapsed CLL and for older and unfit patients in the first-line setting. However, chemoimmunotherapy (CIT) remains the standard-of-care in fit patients. We reported that a subgroup of patients with IGHV mutated CLL experience prolonged PFS and potential cure after first-line CIT withfludarabine, cyclophosphamide and rituximab (FCR). However, FISH data was not available for this cohort of patients. Accurate knowledge of which patients are likely to experience prolonged PFS after FCR is essential to better select patients who may benefit from CIT in the era of novel therapies.
Patients and Methods: We analyzed 492 patients who were treated on six clinical trials of first-line CIT between 2004 and 2015. Treatments were FCR, (n=277) FCR with high dose rituximab (n=65), FCR plusmitoxantrone (n=30), FCR plusalemtuzumab (n=60) and FCR with GM-CSF (n=60). Progression-free survival (PFS) and overall survival (OS) were calculated and pretreatment characteristics were evaluated for association with survival outcomes using a Cox Proportional Hazards model. Cumulative incidence was calculated by competing risk (death without event) regression analysis.
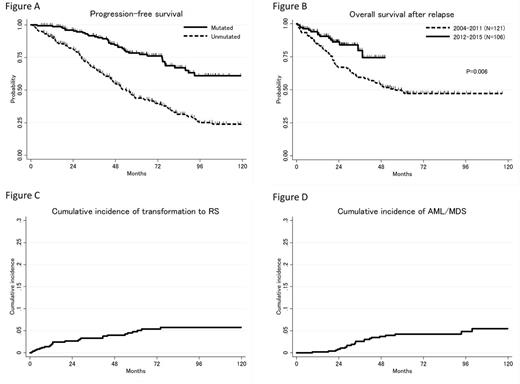
Results: The median age of patients was 59 (range 28-84). Sixty-seven percent of the patients were male, 33% of the patients had mutated IGHV gene. Thirty percent of patients had del(13q), 19% had Trisomy12, 21% had del(11q), 8% had del(17p) and 21% were negative by FISH. Fifty-nine percent of patients received six cycles of CIT. With a median follow up duration of 6.2 years, the median PFS and OS were 6.3 years and not reached, respectively. Recently reported risk model by Rossi and colleagues using IGHV mutation status and FISH results (Blood 2015) discriminated PFS very well; 5-year PFS for low risk {mutated without del(11q)}, intermediate risk {unmutated or del(11q)} and high risk group {del(17p)} were 81%, 45% and 22%, respectively. Of note, there was a plateau in PFS after 8 years in patients with mutated IGHV gene, with 10-year PFS of 63% (Figure A). There was a significantly improved OS after relapse by the time. Three-year OS in patients who started salvage chemotherapy in 2004 to 2012 and 2012 to 2016 were 59% and 83%, respectively, suggesting the impact of improved salvage treatment options, particularly B cell signaling pathway inhibitors (Figure B). Five-year cumulative incidence of Richter transformation (RT) and AML/MDS was 4.8% and 4.2%, respectively (Figure C, D). There was a difference in onset for these two complications; 52% of RT occurred within 2 years, while 62% of AML/MDS occurred in 2-4 years after CIT. Overall, 110 patients (22.4%) died during the follow-up; the three major causes of death were CLL progression (4.9%), Richter transformation (3.7%) and AML/MDS (3.3%).
Conclusion: Patients with mutated IGHV gene and who do not have del(11q) or del(17p) have favorable outcomes and demonstrate a plateau on the PFS curve, consistent with prior studies. Effective salvage therapy has improved outcomes at relapse, but the development of RT and AML/MDS remain major causes of mortality in CLL patients. Given favorable outcomes for patients with mutated IGHV gene treated with FCR, further studies are warranted to identify predictors of non-response among the mutated patients, risk factors for development of AML/MDS and RT and whether choice of first-line therapy can modulate this risk.
Thompson:Pharmacyclics: Consultancy, Honoraria. O'Brien:Janssen: Consultancy, Honoraria; Pharmacyclics, LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding. Jain:Servier: Consultancy, Honoraria; Novimmune: Consultancy, Honoraria; Incyte: Research Funding; Celgene: Research Funding; ADC Therapeutics: Consultancy, Honoraria, Research Funding; Genentech: Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Seattle Genetics: Research Funding; Novartis: Consultancy, Honoraria; Abbvie: Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; BMS: Research Funding; Infinity: Research Funding. Wierda:Abbvie: Research Funding; Novartis: Research Funding; Acerta: Research Funding; Gilead: Research Funding; Genentech: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal