Abstract
Background:
Chronic lymphocytic leukemia (CLL) is primarily a disease of the elderly, with the majority of patients (pts) older than 65 y at diagnosis (US SEER). Historically, younger, more fit pts were eligible for aggressive chemoimmunotherapy treatments, but older age and comorbid conditions can limit options due to tolerability concerns. Ibrutinib (ibr), a first-in-class, oral, once-daily inhibitor of Bruton's tyrosine kinase, is indicated by the US FDA for the treatment of pts with CLL/small lymphocytic lymphoma (SLL) and allows for treatment without chemotherapy. Results from the phase 3 RESONATE (PCYC-1112) and RESONATE-2 (PCYC-1115) trials established ibr as a standard option in relapsed/refractory (R/R) and treatment-naïve (TN) CLL/SLL. This analysis examines safety and efficacy from these studies by age.
Methods:
RESONATE randomized 391 pts with R/R CLL/SLL to receive ibr (n=195; 420 mg once daily) until progressive disease (PD) or unacceptable toxicity or intravenous ofatumumab (n=196; 300 mg at week 1, 2000 mg weekly for 7 w and then every 4 w for 16 w). RESONATE-2 randomized TN pts aged ≥65 y to receive ibr (n=136; 420 mg once daily) until PD or chlorambucil (clb; n=133; 0.5 mg/kg to a maximum of 0.8 mg/kg) on days 1 and 15 of a 28-day cycle for up to 12 cycles. Pt data from both studies were grouped by age (ibr and comparators [comp]) and analyzed for effect of age on outcome.
Results:
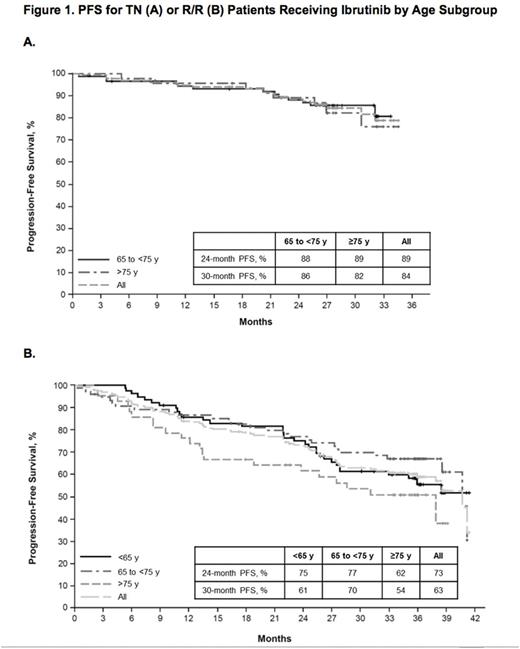
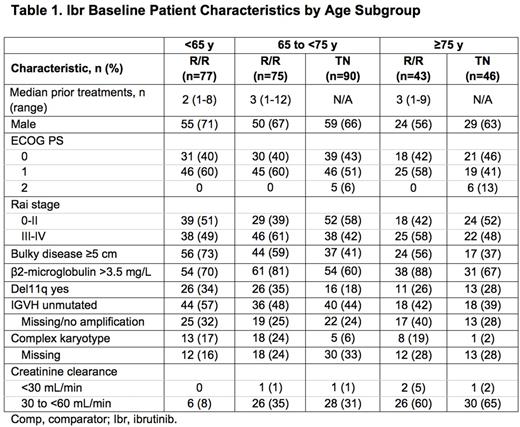
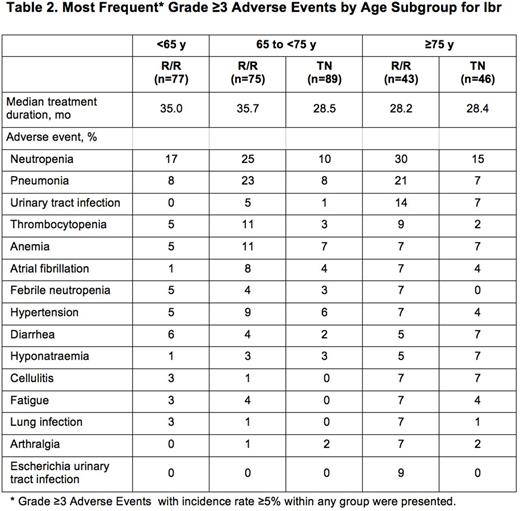
Ibr group included 136 TN pts: 65 to <75 y (termed mid, n=90) and ≥75 y (termed old, n=46), and 195 R/R pts: <65 y (termed young, n=77), 65 to <75 y (mid, n=75), and ≥75 y (old, n=43). Table 1 lists key baseline pt characteristics. For ibr, median follow-up was 28.6 mo for TN and 36.1 mo for R/R pts. PFS is shown in Figure 1. For TN ibr pts, the 24-mo PFS rates were 88% (mid) and 89% (old), whereas clb pts aged ≥75 y did worse than those aged 65 to <75 y (23% vs 40%). For R/R pts, the 24-mo PFS was 75% (young), 77% (mid), and 62% (old). For ibr, median OS was not reached for any age subgroup (R/R or TN). The CR+CRi rates for TN ibr pts were 19% (mid) and 17% (old) and for R/R pts were 6% (young), 9% (mid), and 9% (old). Median treatment duration and the most common grade ≥3 AEs are listed in Table 2. The most frequent any-grade adverse events (AEs) were diarrhea, fatigue, cough, upper respiratory tract infection, nausea, pyrexia, and anemia for all pts and age subgroups. For ibr, grade ≥3 AEs occurred in 64% (mid) and 83% (old) of TN pts, and 70% (young), 84% (mid), and 84% (old) of R/R pts. The prevalence of common grade ≥3 AEs was typically highest in the first 12 mo of ibr therapy, and decreased thereafter for all age groups. Pts ≥75 y had a higher rate of grade ≥3 neutropenia and urinary tract infection in both TN and R/R populations. R/R ibr pts <65 y experienced a lower frequency of grade ≥3 pneumonia and atrial fibrillation (AF) than older subgroups. Any grade AF occurred in 11% (mid) and 9% (old) of TN ibr pts, and 1% (young), 20% (mid), and 14% (old) of R/R ibr pts. For ibr, dose reduction due to AE occurred in 9% (mid) and 22% (old) of TN pts and 6% (young), 20% (mid), and 12% (old) of R/R pts. 107 (79%) TN ibr pts remain on treatment: 81% (mid) and 74% (old); 99 (51%) R/R pts remain on treatment: 49% (young), 59% (mid), and 40% (old). For ibr, discontinuation due to AE occurred in 11% (mid) and 13% (old) of TN pts, and 6% (young), 11% (mid), and 19% (old) of R/R pts. The most common AE leading to discontinuation of ibr was infection(TN: n=4, RR: n=5).
Conclusions:
The PFS rate for TN ibr pts was similar regardless of age subgroup, whereas it was shorter for clb-treated pts ≥75 y compared with those 65 to <75 y. In the R/R population, ibr pts ≥75 y tended to have shorter PFS compared to those <75 y. There was no perceivable difference in CR rate by age within the TN and R/R ibr populations. The rates of grade ≥3 AEs tended to be higher in pts ≥75 y for both ibr and comp arms. Age did not impact discontinuation rates due to AE for TN pts treated with ibr, but increased with age for R/R pts. This study is limited by the number of pts ≤65 y who were only eligible for the R/R trial. This analysis supports single-agent ibrutinib as a treatment option regardless of pt age, but reinforces the need for close toxicity monitoring, especially in older pts.
Woyach:Karyopharm: Research Funding; Morphosys: Research Funding; Acerta: Research Funding. Hillmen:Pharmacyclics: Research Funding; Janssen: Honoraria, Research Funding; Roche: Honoraria, Research Funding; Gilead: Honoraria, Research Funding; Abbvie: Research Funding. Brown:Pfizer: Consultancy, Honoraria, Other: travel, accommodations, expenses; Genentech: Consultancy, Honoraria; Roche: Honoraria; Infinity: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Other: travel, accommodations, expenses; Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Other: travel, accommodations, expenses; Pharmacyclics, LLC, an AbbVie Company: Consultancy, Honoraria; Sun Biopharma: Honoraria, Other: travel, accommodations, or other expenses. Coutre:Pharmacyclics, LLC, an AbbVie Company: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; AbbVie: Research Funding. Barr:AbbVie: Consultancy; Pharmacyclics, LLC, an AbbVie Company: Consultancy, Research Funding. O'Brien:Janssen: Consultancy, Honoraria; Pharmacyclics, LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding. Devereux:Janssen: Consultancy, Other: Travel, Accommodations, Expenses, Speakers Bureau; GSK: Consultancy; Roche: Consultancy, Other: Travel, Accommodations, Expenses ; Gilead: Consultancy, Other: Travel, Accommodations, Expenses, Speakers Bureau. Reddy:KITE: Membership on an entity's Board of Directors or advisory committees; celgene: Membership on an entity's Board of Directors or advisory committees; GILEAD: Membership on an entity's Board of Directors or advisory committees; INFINITY: Membership on an entity's Board of Directors or advisory committees. Mulligan:GSK: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria, Research Funding, Speakers Bureau. Zhou:AbbVie: Equity Ownership; Pharmacyclics, LLC, an AbbVie Company: Employment. Ninomoto:AbbVie: Equity Ownership; Amgen: Equity Ownership; Pharmacyclics, LLC, an AbbVie Company: Employment. James:Pharmacyclics, LLC, an AbbVie Company: Employment; AbbVie: Equity Ownership. Burger:Janssen: Consultancy, Other: Travel, Accommodations, Expenses; Portola: Consultancy; Gilead: Research Funding; Pharmacyclics, LLC, an AbbVie Company: Research Funding; Roche: Other: Travel, Accommodations, Expenses.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal