Abstract
Background: DNMT3A mutations result in epigenetic dysregulation and impart a negative prognostic impact in acute myeloid leukemia and myelodysplastic syndromes. In chronic myelomonocytic leukemia (CMML), DNMT3A mutations are seen in 2-5% of patients. In a large Groupe Français des Myélodysplasies (GFM) study (n=312), DNMT3A mutations were seen in 2% and were not included in further survival analyses (Itzykson JCO 2013). In a prior Mayo Clinic study (n=175), DNMT3A mutations were seen in 5% (n=9) and on univariate, but not multivariate analysis (Patnaik Blood C J 2016), were associated with shortened over-all survival (OS). We carried out this study on a larger CMML cohort (n=261), with more (n=15) informative cases to assess the impact of DNMT3A mutations.
Methods: 261 patients with World Health Organization (WHO)-defined CMML were included in the study. All patients had bone marrow (BM) biopsies and cytogenetics performed at diagnosis. Targeted capture assays were carried out on BM DNA specimens obtained at diagnosis for the following genes; TET2, DNMT3A, IDH1, IDH2, ASXL1, EZH2, SUZ12, SRSF2, SF3B1, ZRSR2, U2AF1, PTPN11, Tp53, SH2B3, RUNX1, CBL, NRAS, KRAS, JAK2, CSF3R, FLT3, KIT, CALR, MPL, NPM1, CEBPA, IKZF, and SETBP1. The 2016 WHO diagnostic criteria were used.
Results:Among the 261 study patients, 65% were males and median age was 70 years (range, 28-91). 154 (59%), 64 (25%) and 43 (16%) patients were classified as CMML-0, 1 and 2, respectively. At a median follow-up of 23 months, 174 (67%) deaths and 37 (14%) leukemic transformations (LT) were documented. Mutational frequencies ≥4% were encountered in; TET2 45%, ASXL1 45%, SRSF2 40%, NRAS 14%, SETBP1 13%, CBL 10%, JAK2 7%, RUNX1 6%, DNMT3A 6%, U2AF1 6%, SF3B1 5%, ZRSR2 4%, Tp53 4%, and IDH2 4%.
i) DNTM3A mutated CMML: phenotypic and molecular correlates
DNMT3A mutations were seen in 15 (6%) patients; 64% male with a median age of 64 years. DNMT3A amino acid substitutions included; R882H 50%, R882C 29%, R910P 7%, R598* 7% and R320* 7%. The median variant allele frequency burden was 45%. Concurrent gene mutations were detected in; TET2 43%, ASXL1 21%, SF3B1 21%, U2AF1 14%, RUNX1 14%, SETBP1 14%, NRAS 14%, SRSF2 7%, JAK2 7% and Tp53 7%. There was no difference between DNMT3A mutated and wild-type patients in terms of age and gender distribution, hemoglobin level, leukocyte, monocyte (AMC), and platelet counts, peripheral blood (PB) or BM blast content. Concurrent gene mutations were equally distributed with the exception for a higher prevalence of SF3B1 (p=0.003) and a lower prevalence of SRSF2 (p=0.004) mutations in DNMT3A mutated CMML. Four (29%) patients underwent leukemic transformation.
ii) Impact on OS and leukemia-free survival (LFS):
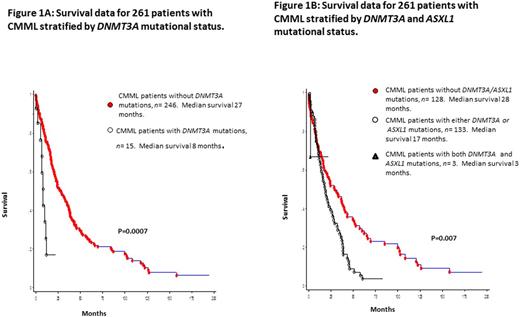
Median survival for the entire cohort (n=261) was 24 months. In univariate analysis, survival was shorter in DNMT3A mutated (median 8 months) versus wild-type (median 27 months) patients (p=0.0007; HR 2.9, 95% CI 1.5-5.7; Figure 1A). Other variables of significance, in univariate analysis, included lower hemoglobin (p=0.002), higher leukocyte count (p=0.0009), higher AMC (p=0.0012), PB blast % (p=0.001), circulating immature myeloid cells (IMC, p=0.01), BM blast % (p=0.045), abnormal karyotype (p=0.02), and ASXL1 (p=0.01) mutations. Survival was also adversely affected by the presence of either (n=133) or both (n=3) ASXL1/DNMT3A mutations (0=0.007, Figure 1B). In multivariable analysis (MVA) excluding ASXL1 and DNMT3A mutations, hemoglobin (p=0.03), IMC (p=0.013) and AMC (p=0.02) retained significance. When ASXL1 mutations were added to the MVA, ASXL1 (p=0.01) mutations, AMC (p=0.012) and IMC (p=0.03) retained significance. Similarly, when only DNMT3A mutations were added to the MVA, DNMT3A (p=0.003) mutations, IMC (p=0.01) and AMC (p=0.02) retained significance. When both DNMT3A and ASXL1 mutations were added to the MVA, only DNMT3A (p<0.0001) and ASXL1 (p=0.004) mutations remained significant. DNMT3A mutations predicted shortened OS, independent of the ASXL1 inclusive GFM model (p<0.0001) and Mayo Molecular Model (p=0.002). DNMT3A mutations (p=0.0018), along with low hemoglobin levels (p=0.003) independently predicted for a shorter LFS.
Conclusions:DNMT3A mutations are seen in ~5% of patients with CMML and impart a negative prognostic impact on both OS and LFS. This finding warrants inclusion of DNMT3A mutations in molecularly integrated CMML prognostic models.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal