Abstract
Background Hypomethylating agent (HMA) therapy represents the standard of care for patients with higher risk myelodysplastic syndromes although only 50% of patients respond to treatment. Recent evidence from molecular profiling through next-generation sequencing (NGS) in myeloid diseases has been conflicting as to the value of somatic mutations as a biomarker for response to HMA. TET2 mutations, predominantly in the absence of ASXL1 mutations (or variant allele frequency (VAF) < 10%), have been shown to predict HMA response in MDS and chronic myelomonocytic leukemia (CMML) (Itzykson et al., 2011; Bejar et al., 2014 (80% decitabine)) while DNMT3A mutations predict response to frontline HMA treatment in acute myeloid leukemia (AML) (Coombs et al., 2016). Therefore, our goal was to identify molecular predictors of response and outcomes to azacitidine in myeloid malignancies.
Patients and MethodsGenetically profiled higher-risk MDS, CMML and oligoblastic AML (20-30% blasts) cases were retrospectively identified from the Moffitt Cancer Center MDS database. We evaluated gene mutations associated with DNA methylation (TET2, DNMT3A, IDH1, IDH2, and WT1) and up to 19 additional genes. NGS was performed prior to the initiation of HMA in all patients. The lower limit of VAF detection was set at 5% and the minimum depth of coverage at each position was 500X. Clinical variables and outcomes of MDS patients were characterized at the time of sample procurement. Fisher's exact and t-tests were used for comparative analyses. Kaplan-Meier curves were used to estimate overall survival and analyzed from the date of mutation identification. Multivariate Cox regression models were created to adjust for clinical characteristics.
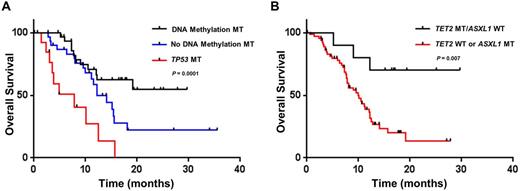
Results From May 2013 to February 2016, a total of 77 patients with NGS for somatic mutations prior to HMA therapy were identified with a median age of 70 years and male predominance (66%). Of the cohort, 97% of patients (n=75) were treated with azacitidine with 17% of patients (n=13) proceeding to allogeneic hematopoietic stem cell transplant (AHSCT). A total of 86% of patients (n=66) had at least one pathogenic mutation. Mutations in DNA methylation occurred in 43% of patients (n=33) while TET2 mutation without clonal ASXL1 mutations occurred in 13% of patients (n=10). At a median follow up 17 months, the median OS of the entire cohort was 12.5 months. Patients with a DNA methylation mutation had a median OS that was not reached (NR) vs a median OS of 11.5 months in wildtype (WT) patients (HR 0.38, 95% CI 0.21 to 0.75; P = 0.005), which remained significant when censoring patients at time of AHSCT (P = 0.002). TP53 mutant (MT) patients (n=14, 18% of cohort) had a median OS of 7.9 months vs 15.4 months in WT patients (HR 3.69, 95% CI 3.04 to 28.8; P = 0.0001). The presence of DNA methylation or TP53 mutation in comparison to wildtype patients significantly stratified prognosis in azacitidine treated patients (Figure 1A, P = 0.0001). In multivariable analysis incorporating age and revised international prognostic scoring system (IPSS-R) category, DNA methylation (HR 0.45, 95% CI 0.21 to 0.96; P = 0.04) and TP53 (HR 2.34, 95% CI 1.04 to 5.26; P = 0.04) mutation status remained predictive for survival. Notably, response rates in DNA methylation mutant patients were similar to WT patients (40% versus 42%) with no difference in treatment duration. However, the overall response rate of TET2 MT/ASXL1 WT patients was 70% versus 36% in the rest of the cohort (P =0.08) and 0% in TET2 MT/ASXL1 MT patients (0/6, P =0.01) with a significantly longer duration of treatment in the TET2 MT/ASXL1 WT cohort (median number of cycles 7.5 versus 4; P = 0.002). Additionally, TET2 MT/ASXL1 WT patients had longer survival (median OS NR vs 12.2 months; P = 0.047). When censoring for transplant, the impact of TET2 MT/ASXL1 WT genoptype was significantly strengthened with 70% of patients alive past 15 months (median OS NR vs 9.9 months; HR 0.24, 95% CI 0.19 to 0.75; P = 0.007; Figure 1B).
Conclusion In patients with myeloid malignancies, molecular profiling via NGS can predict outcomes to azacitidine therapy. Patients with mutations of DNA methylation have improved OS whereas OS is poor in TP53 MT patients. Most importantly, TET2 MT/ASXL1 WT identifies a genotypic subgroup with particularly good outcomes when treated with HMA without AHSCT and potentially challenges the early timing of AHSCT for higher risk patients and this molecular profile.
Padron:Novartis: Honoraria; Incyte: Research Funding; CTI: Honoraria, Research Funding; KALOBIOS: Research Funding. Vaupel:Genoptix, a Novartis Company: Employment. Hall:Genoptix, a Novartis Company: Employment. Komrokji:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Speakers Bureau; Boehringer-Ingelheim: Research Funding; Incyte: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal