Abstract
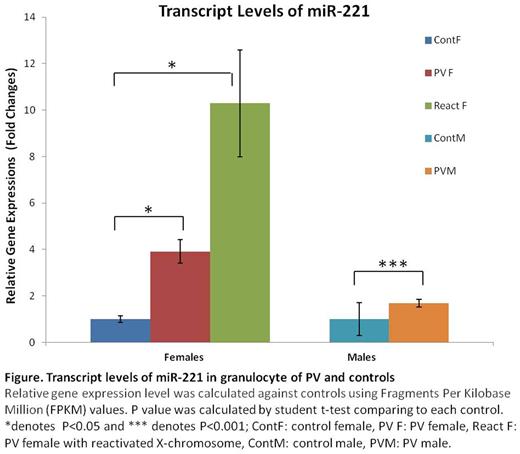
First and second authors deserve equal credit In each female somatic cell, most genes on either the maternal or paternal X-chromosomes are randomly inactivated at early embryogenesis. This remains constant in the progeny of these cells throughout life. The XIST gene is a principal regulator of X-chromosome inactivation expressed in inactive female X-chromosomes. It encodes a long noncoding RNA which leads to recruitment of histone H3 lysine 27 trimethylation (H3K27me3)-methylase, and eventual inactivation of transcripts of most X-chromosome genes on inactive X-chromosomes. The clonality of human tumors is central to our understanding of malignant and premalignant diseases such as polycythemia vera (PV) and essential thrombocythemia (ET). Clonal populations can be detected by expression of X-chromosome genes in females. In a normal female embryo, there are eight cells at the time of random embryonic X-chromosome inactivation; thus X-chromosome allelic ratios vary from female to female. This ratio is stable in time, identical in all blood cell lineages, and the same for any interrogated X-linked gene in females heterozygous for other X-chromosome inactivated genes (Prchal, J Exp Med, 1996). Based on polymorphisms for 5 X-chromosome genes (MPP1, FHL1, IDS, BTK, and G6PD), we developed a quantitative transcriptional clonality assay and found that allelic usage ratios are the same for each marker and in each myeloid cell line (Swierczek, Blood 2009). In two PV (P1, P3) and one ET (P2) females, we observed expression of both alleles of either the IDS or G6PD genes in platelets and granulocytes, whereas other X-chromosome genes for which these women were heterozygous were expressed only a single allele. We then analyzed their X- chromosome transcripts in clonogenic assays of early erythroid progenitors (BFU-E). Each of these females had some BFU-E which expressed both X-chromosome genes. Reactivation of inactivated X-chromosomes was reported in some human breast cancers (Richardson, Cancer Cell, 2006) and was associated with overexpression of a small subset of X-chromosomal oncogenes and/or tumor suppressor genes (Thakur, Mole Cancer Res, 2007). Further, conditional Xist deletion inhematopoietic stem cells of female mice resulted in global X-chromosome reactivation and complete penetrance of a highly aggressive PV/ET-like syndrome that progressed to fatal lymphoma, acute leukemia or myelodysplastic syndrome (Yildirim, Cell,2013). Our previous, unbiased, whole exome sequencing of 31 JAK2V617F positive PV patients identified 87 somatic and germline mutations, with most patients having two or more somatic mutations (Wang, Leukemia, 2014). The majority of mutated genes were epigenetic modifiers; i.e. ASXL1, DNMT3A, TET2, SF3B1 and PDE4C. We interrogated the effects of epigenetic modifiers on methylation of DNA of CD34 cells and granulocytes by whole genome methylome analysis and demonstrated inconsistent, scattered hypomethylation changes in autosomes of both males and females. However, there were large regions of the hypomethylated X-chromosome genome in many but not all PV and ET females. We examined transcript levels of the hypomethylated genes by QT-RTPCR and found augmentation of transcripts of many but not all hypomethylated genes in granulocytes and platelets in these PV females. However, XIST transcripts were not decreased. The ChIP analysis of clonal granulocytes revealed decreased level of H3K27me3 at hypomethylated regions of biallelically expressed G6PD and IDS genes. We then performed unbiased transcriptome analysis by RNA seq of 24 PV and ET males and females (Figure). We observed more overexpressed genes (75%) on X chromosome than autosomes (66%).The X-cromosome encoded miR-221 (reported dysregulated in ET; Navarro, Blood Cancer Journal, 2016) and ARHGAP6 were overexpressed in PV and ET but more in females than males (Figure). The miR-221 targets SOCS1 and SOCS3 genes, negative regulators of the JAK2/STAT pathway, and the ARHGAP6 gene is a component of PDGF signaling pathway.
It remains to be determined which hypomethylated X-chromosome genomic regions have functional activity in hematopoiesis in some PV females but miR-221 and ARHGAP6 are potential candidates. These data may contribute to a better understanding of gender differences of the PV phenotype and PV morbidity and mortality. One such an example is higher prevalence of Budd-Chiari syndrome in females.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal