Abstract
Ruxolitinib is an orally available JAK1/2 inhibitor FDA approved for the treatment of myelofibrosis and polycythemia vera. Evidence suggest the effect of ruxolitinib on IL-1, IL-6, and TNF-α, among other immune mediators, may predispose patients to both opportunistic and non-opportunistic infections. Identification of patients at risk for infection while on a JAK inhibitor may impact clinical management. Authors sought to determine the cumulative incidence of ruxolitinib-related infections in the clinical practice setting and evaluated patient factors in order to characterize the types, onset, and severity of infections.
This IRB approved, single-center, retrospective study at Memorial Sloan Kettering Cancer Center included myeloproliferative neoplasm (MPN) patients treated with ruxolitinib who were ≥18 years of age between 12/1/11 and 12/31/15. Patients on ruxolitinib for less than seven days, receiving ruxolitinib therapy for a non-MPN diagnosis, or had a manifestation of acute leukemia were excluded. Patients who met inclusion criteria were censored at time of splenectomy and hematopoietic stem cell transplant. Patients were identified through electronic medical records and an internal pharmacy database. Cumulative incidence of treatment-related infection was estimated and association with factors was assessed using competing risk regression analysis. Death without an infection was considered a competing risk event.
A total of 44 patients were included in this analysis. The median age of this cohort was 66.5 years, with a slightly predominant male population (57%). There were 19 (43%) primary and 19 (43%) secondary myelofibrosis patients, 4 (9%) with polycythemia vera, and 2 (5%) patients with an unclassifiable MPN. For myelofibrosis patients, according to the Dynamic International Prognostic Scoring System (DIPSS), 2 (5%) were low risk, 3 (7%) were intermediate-1, 16 (36%) were intermediate-2, and 7 (16%) were high; remaining patients did not have available DIPSS scores at the time of evaluation. The median number of prior lines of therapy was 1.5 (range 0-4); median total daily dose of ruxolitinib was 10 mg. A majority of patients (70%) were not on concomitant immunosuppressive therapy during ruxolitinib treatment or strong CYP3A4 inhibitors (95%) that may have increased exposure to ruxolitinib.
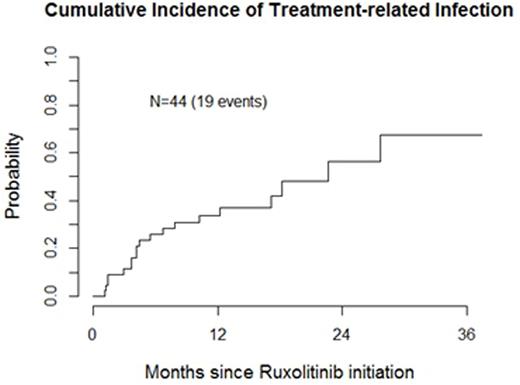
A total of 20 documented infections were identified throughout the evaluation period; median follow-up among survivors was 16 months (range 3-51). Cumulative incidences of treatment related infection were 33% by year 1 and 56% by year 2 of ruxolitinib treatment. Of these infections, four were CTCAEv4.0 grade 1 (one asymptomatic upper respiratory tract infection (URI) occurred after discontinuation was censored), seven were grade 2, and eight were grade 3. One grade 5 infection, septic shock of unknown origin occurred. Only one opportunistic pulmonary tuberculosis infection occurred 13 months post ruxolitinib treatment. The remainder of infections varied, including URIs(n=9), pneumonia, diverticulitis, osteomyelitis, clostridium difficile, proctitis, catheter-associated UTI, dental abscess, herpes zoster activation of the skin, and oral herpes simplex reactivation (all n=1)). One patient required viral prophylaxis for recurrent herpes simplex infections.
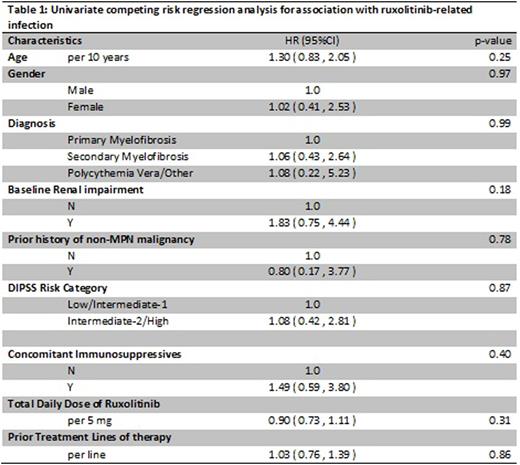
With the limited number of events, no factors were found to be significantly associated with increased risk of infection but a trend toward increased risk of ruxolitinib-related infection was observed with baseline renal impairment. (Table 1).
Conclusion: This data suggests a higher incidence of ruxolitinib associated infections observed in clinical practice compared to rates described in trials of ruxolitinib therapy. This may be attributable to differences between populations in the COMFORT and RESPONSE trials and patients in the clinical practice setting. Generally, infections were non-life threatening and managed with appropriate supportive care. Plasma values of ruxolitinib metabolites increase as renal function declines, correlation of infection risk via increased metabolites exposure warrants further exploration. Routine antimicrobial prophylaxis for all patients receiving ruxolitinib therapy is likely not warranted. However larger studies are needed to confirm these observations.
Mauro:Pfizer: Consultancy; Ariad: Consultancy; BMS: Consultancy; Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal