Abstract
Background: ENESTop (NCT01698905) is an ongoing, single-arm, phase 2 study evaluating treatment-free remission (TFR) in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) who achieved sustained molecular response 4.5 (MR4.5; BCR-ABL1 ≤ 0.0032% on the International Scale [BCR-ABL1IS]) on second-line nilotinib (NIL). In the primary analysis, 57.9% (95% CI, 48.8%-66.7%) of pts who stopped NIL maintained TFR (no loss of major molecular response [MMR; ie, BCR-ABL1IS > 0.1%], confirmed loss of MR4 [ie, consecutive BCR-ABL1IS > 0.01%], or treatment reinitiation) at 48 weeks.
Adverse events (AEs) were reported in 73.8% of pts during the first 48 weeks of TFR vs 77.0% during the year prior to stopping treatment. The incidence of musculoskeletal pain-related AEs was higher during TFR (42.1% vs 14.3%). To assess the potential effect of stopping NIL on quality of life (QOL), pt-reported outcomes were assessed before and during TFR.
Methods: ENESTop enrolled adult pts with CML-CP with ≥ 3 years of prior tyrosine kinase inhibitor (TKI) therapy (> 4 weeks of imatinib [IM], then ≥ 2 years of NIL). Pts must have achieved sustained MR4.5 on NIL after switching from IM. Enrolled pts entered a 1-year NIL treatment consolidation phase; those without confirmed loss of MR4.5 (ie, consecutive BCR-ABL1IS > 0.0032%) entered the TFR phase and stopped NIL. Pts with loss of MMR or confirmed loss of MR4 during the TFR phase reinitiated NIL.
QOL was assessed via questionnaires completed by pts at specified time points. The MD Anderson Symptom Inventory for CML (MDASI-CML) questionnaire assessed, for a defined set of symptoms, the levels of severity and interference with daily life on a scale of 0 to 10, with 0 being the lowest level. The EQ-5D-5L questionnaire assessed problems experienced by pts (no, slight, moderate, severe, or extreme [ie, interfering with functioning] problems) in 5 dimensions: mobility, self-care, usual activities, anxiety/depression, and pain/discomfort; additionally, overall level of health was assessed on a scale of 0 to 100 using the EQ VAS, with 0 being the lowest level.
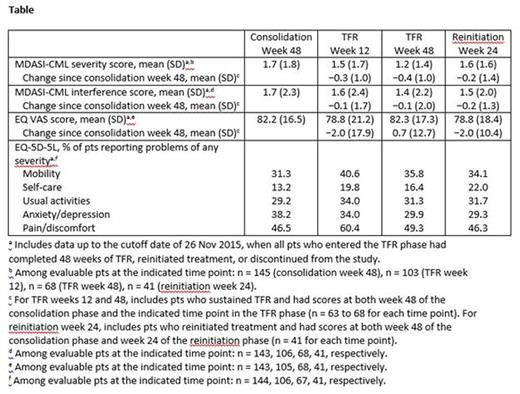
Results: Of 163 enrolled pts, 126 met the criteria for stopping NIL in the TFR phase; of these 126, 53 had loss of MMR or confirmed loss of MR4, of whom 51 reinitiated NIL. Among pts who completed each questionnaire at week 48 of the consolidation phase, week 12 of the TFR phase, or week 48 of the TFR phase, mean MDASI-CML severity scores were 1.7, 1.5, and 1.2, respectively; mean MDASI-CML interference scores were 1.7, 1.6, and 1.4, respectively; and mean EQ VAS scores were 82.2, 78.8, and 82.3, respectively (Table). Among pts with sustained TFR and scores at both week 48 of the consolidation phase and week 12 or 48 of the TFR phase, minimal changes were observed in MDASI-CML and EQ VAS scores during TFR vs week 48 of the consolidation phase. Among pts who reinitiated NIL, mean MDASI-CML severity and interference scores and mean EQ VAS scores at 24 weeks after the start of retreatment were 1.6, 1.5, and 78.8, respectively; changes in scores between week 24 after treatment reinitiation and week 48 of the consolidation phase were minimal among pts with scores at both time points.
Among pts who completed the EQ-5D-5L questionnaire, the proportions who reported problems (any level of severity) in the 5 dimensions were generally similar across time points, although problems with mobility and pain/discomfort were more common during TFR vs week 48 of the consolidation phase, particularly at week 12 of the TFR phase (Table).
Conclusion: Changes in QOL after stopping NIL, as assessed using these questionnaires, were minimal. This may be related to pts having a relatively high QOL prior to stopping treatment given that they had tolerated ≥ 3 years of TKI therapy, including ≥ 2 years of NIL, prior to enrollment. Despite the higher incidence of musculoskeletal pain-related AEs observed during TFR (42.1% vs 14.3%), the overall QOL was generally similar between the TFR and consolidation phases. This suggests that these AEs did not have a significant negative impact on QOL. Longer follow-up in ENESTop will be needed to further evaluate trends in QOL after stopping second-line NIL.
Mahon:NOVARTIS PHARMA: Honoraria, Research Funding; BMS: Honoraria; ARIAD: Honoraria; PFIZER: Honoraria. Boquimpani:BMS: Speakers Bureau; Novartis: Research Funding, Speakers Bureau. Takahashi:Novartis: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; BMS: Honoraria. Shuvaev:Novartis pharma: Honoraria; Pfizer: Honoraria; BMS: Honoraria. Ailawadhi:Pharmacyclics: Consultancy; Novartis: Consultancy; Amgen Inc: Consultancy; Takeda Oncology: Consultancy. Lipton:Novartis: Consultancy, Research Funding; Ariad: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding. Turkina:Novartis Pharma: Honoraria; Pfizer: Honoraria; BMS: Honoraria. Moiraghi:NOVARTIS: Speakers Bureau; BMS: Speakers Bureau. Nicolini:Ariad: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria. Sacha:Pfizer: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Adamed: Consultancy, Honoraria. Kim:BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; ILYANG: Consultancy, Honoraria, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau. Fellague-Chebra:Novartis: Employment. Acharya:Novartis Healthcare Pvt. Ltd.: Employment. Brandt:Novartis: Employment. Gnanasakthy:Novartis: Consultancy, Equity Ownership, Research Funding. Jin:Novartis: Employment, Equity Ownership. Hughes:Ariad: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal