Abstract
Introduction:
Diffuse large B-Cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma (NHL) in HIV population and the survival rate at 2 years is still only 50% (Olszewski AJ et al, Cancer 2016). Based on the cell-of-origin (COO) by immunohistochemistry (IHC), DLBCL is classified into germinal center B-cell like (GCB) and activated B-cell like (ABC or non-GCB) subtypes. While it is well accepted that the ABC subtype is associated with poor outcome in non-HIV DLBCL, the prognostic value of the COO classification in HIV DLBCL has been an issue of debate (Chadburn et al JCO 2009, Dunleavy et al 2010). Current clinical trials stratify patients based on COO in non-HIV DLBCL as the ABC subtype responds better to novel agents like Ibrutinib than GCB. Hence it is important to know whether the two subtypes retain their biological differences in HIV related DLBCL. In order to understand the effect of prognostic variables including subtype and treatment, on survival we conducted a retrospective review of HIV DLBCL in a large single center cohort.
Methods:
We conducted a retrospective analysis of all HIV infected patients diagnosed with DLBCL (excluding primary CNS) at Montefiore Medical Center between 2000- 2014. The variables that were utilized in the analysis are time of diagnosis of DLBCL, stage, COO subtype of DLBCL (GCB vs non-GCB based on Hans method), LDH (high = >391 u/L), extra-nodal involvement, CD4 count at diagnosis, combination anti-retroviral therapy (cART) at diagnosis, type of chemotherapy, use of rituximab and survival status. Time was divided in 5-year increments -2000 to 2005 (E1), 2005 to 2010 (E2) and 2010 to 2014 (E3).
Results:
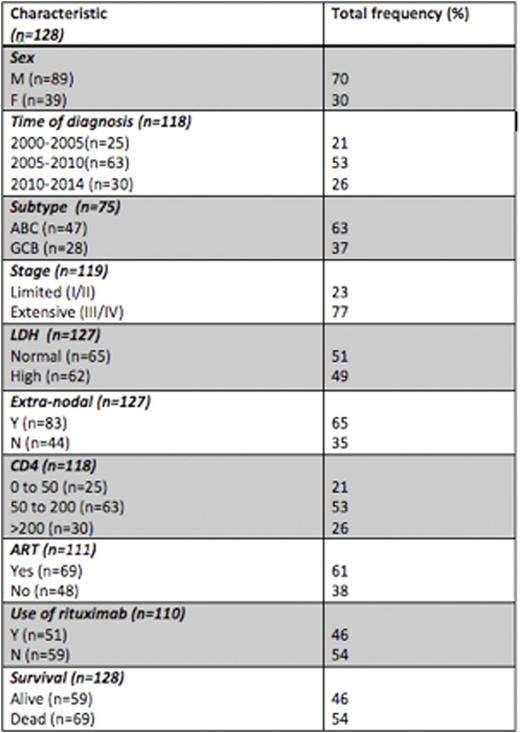
A total of 128 patients with HIV-DLBCL were identified. The mean age was 47 years with a male predominance (70%). Features of high-risk disease were advanced stage III/IV (77%), presence of extra-nodal involvement (65%), and high LDH (51%) seen in 87% of patients. COO classification by IHC (n=75) showed that most were non-GCB (63%) and the rest were GCB (37%) phenotype. HIV specific factors at diagnosis were -CD4 0 to 50 cells (21%), 50-200 (53%) and >200 (25%), mean HIV viral load of 16,459 copies/ml and 61% were on cART. Overall 89% of all patients received treatment and 82% received combination chemotherapy with anthracyclines. There was no significant association between rituximab use (46%) and CD4 counts at diagnosis (p=0.24) while rituximab use was higher in E3 than E1 time period, as expected (p=0.01).
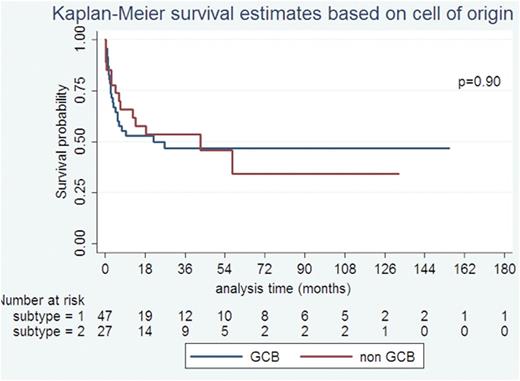
The median OS was 26.6 months. On univariate analysis, high LDH (p=0.03), not receiving rituximab (p=0.01) and extensive stage disease (p= 0.034) were significantly associated with poor OS. There was no difference in survival between the 3 time periods (p=0.96). COO subtypes (non-GCB vs GCB) was not predictive of OS (P=0.90), as were HIV specific factors at diagnosis -CD4 (p=0.88) and ART (p=0.51). Cox proportional hazards model showed only rituximab use (HR=0.48 p=0.02) as an independent predictor of survival but not age (HR=1.02, p=0.09), stage (HR=1.95, p=0.09), LDH (HR=1.37, p=0.31) and extra-nodal disease (HR=1.67, p=0.1). Rituximab was the only independent predictor even when subtype was included in the model (n=58) or when restricted to the subset that received treatment (n=94).
Conclusions:
This is one of the largest cohorts of HIV-DLBCL analyzed. This study shows that the median age of HIV DLBCL is lower, presents with high-risk disease -higher stage, extra nodal involvement and high LDH. HIV specific risk factors like ART and CD4 counts at diagnosis were not prognostic in the era of cART. Even though stage, LDH and extranodal involvement were significant in the univariate analysis, only rituximab use was independently associated with OS in the multivariate analysis. Rituximab use was not significantly different based on CD4 counts, whilst there was an increase in use with time; OS was not significantly different between the time periods. These findings show that rituximab should be used in all HIV DLBCL independent of CD4 counts.
The other important finding is that the COO sub classification was not predictive of prognosis in contrast to non HIV-DLBCL where the ABC subtype has an inferior prognosis. Higher expression of c-myc present in HIV-DLBCL may negate the better prognosis of GCB subtype (Chao C et al, CCR 2015). This needs to be explored further, new pathological prognostic markers are to be identified to risk stratify patients and therapeutic subgroups.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal