Abstract
Introduction
CXD101 is a class I-selective HDAC inhibitor (HDACi)with no demonstrable activity against HDAC class II in non-clinical studies. CXD101 induces specific HDAC inhibition and subsequent to that, enhanced histone acetylation in a time and dose-dependent fashion in cell lines cells in vitro, leucocytes obtained from treated rats and dogs, and human leucocytes treated ex vivo. CXD101 has been tested on several tumor cell lines including non-Hodgkin lymphoma (NHL) and myeloma.
We sought to determine the maximal tolerated dose (MTD) of CXD101 in a dose escalation study in patients with advanced solid and haematological cancer.We report the phase 1 first-in-human experience with CXD101 in patients with advanced malignancy.
Methods
In this phase 1 study we escalated the dose of CXD101 from 1 mgadministered orally twice daily (B.D.)for 5 days in a 21 day cycle, following a 3+3 design. Eligible patients had advanced solid tumours or relapsed, refractory lymphoma. A preliminary assessment of efficacy was performed in a dose expansion cohort after the MTD was found. 39 patients were enrolled, of whom 36 were exposed to CXD101. Lymphoma patients were assessed by Cheson 2007 criteria, and solid tumor patients by RESIST 1.1.
A genome-wide loss-of-function screen identified HR23B, a protein that shuttles ubiquitinated cargo proteins to the proteasome, as a sensitivity determinant for HDAC inhibitor-induced cell apoptosis(Fotheringham et al., 2009).HR23B status was assessed in all cases with available archival tumor tissue.
Results
MTD
Thirty patients were enrolled in the dose escalation cohort of whom 29 were evaluable for DLT assessment. The first DLT was noted at 16mg B.D. in cohort 5 (QTc prolongation). Subsequent DLTs were seen at 20mg B.D. (1 of 6; grade 4 neutropenia), and at the non-tolerated dose of 24/25mg B.D. (2 of 5; both neutropenic infection). A formal MTD and recommended phase II dose was established at 20 mg B.D. This dose was taken forward to treat 6 patients within the expansion cohort to date.
Lymphoma patients - efficacy
The baseline characteristics of 22 lymphoma patients included amedian age was53.5 years (range 21-79 years) and 59% were male. Patients received a median of 4 prior lines of therapy. The international prognostic index was 3-5 in 85%.
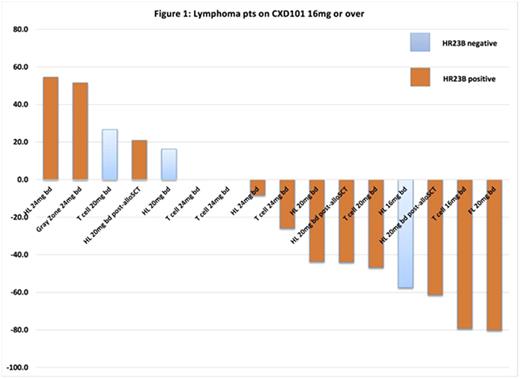
In the 17 lymphoma patients dosed at 16mg b.d. or above, 3 partial responses (2 Hodgkin lymphoma post allogenic stem cell transplantation, 1 refractory angioimmunoblastic T-cell lymphoma) and 1 complete response (relapsed follicular lymphoma) were noted (overall response rate 23.5%) alongside 7 patients gaining clinical benefit with stable disease. Tumor volume reduction was seen in 56% of lymphoma cases (Figure 1). The median progression free survival in these 17 patients was 113 days (95% confidence interval (CI) 35-162 days). In those with PR (n=3) and CR (n=1), the duration of response was: 203 days, 161 days, 141 days (ongoing), and 176 days (ongoing) respectively.Detailed results on the solid tumor patients are reported separately (ESMO, 2016).
Safety
CXD101 was typically well tolerated. Across all 36 patients, possibly, probably or definitely IMP-relatedadverse events (AE) occurring in >10% of the total cycles doses regardless of attribution were: thrombocytopenia (28% grade 1-2; 10% grade 3-4), neutropenia (20% grade 1-2; 16% grade 3-4), anaemia (10% grade 1-2), nausea (19% grade 1-2), diarrhoea (11% grade 1-2), vomiting (11% grade 1-2), anorexia (10% grade 1-2), fatigue (25% grade 1-2), QTc prolongation (17% grade 1-2). The most frequent AEs observed were consistent with the known AE prolife of HDACi. All AEs were manageable.
Conclusions
CXD101 demonstrates a favourable safety profile. The MTD for CXD101 observed was 20 mg B.D. for 5 days in a 3-weekly cycle. Disease activity was seen in T-cell lymphoma, Follicular lymphoma and Hodgkin lymphoma (including post-allogenic stem cell transplantation). Further analysis of HR23B correlated tumor response and durability is ongoing and will be presented alongside pharmacokinetic and pharmacodynamic data.
Trial ID: NCT01977638.
Eyre:Gilead: Honoraria, Other: Travel, Accomodation, Speakers Bureau; Celgene: Other: Travel, Accomodation; GSK: Honoraria; Takeda: Honoraria, Other: Travel, Speakers Bureau. Collins:Takeda: Consultancy, Honoraria, Speakers Bureau. Whittaker:Celleron Therapeutics: Employment, Equity Ownership. La Thangue:Celleron: Employment, Equity Ownership. Kerr:Celleron Therapeutics: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal