Abstract
Background: Follicular lymphoma (FL) is the most common subtype of indolent NHL. Current chemoimmunotherapeutic regimens used for FL are not curative. Rituximab (RTX), as a single-agent and in combination with chemotherapy, is a commonly used frontline approach for FL. Ibrutinib (ibr), a first-in-class, once-daily, oral inhibitor of Bruton's tyrosine kinase, showed activity in a phase 1, dose-escalation study in patients (pts) with relapsed/refractory FL, with an overall response rate (ORR) of 38% (Advani, J Clin Oncol 2013). In this phase 2 multicenter, open-label study of ibr combined with RTX, an ORR of 82% and a complete response [CR] rate of 27% for Arm 1 at a median follow-up of 10.2 months (mo) was previously reported (Fowler, ASH 2015). Here, current efficacy and safety results of this study for Arms 1 and 2 using 2 different treatment combination schedules are presented.
Methods: Pts with treatment-naïve, histologically confirmed FL (grade 1, 2 and 3a, stage II-IV disease) were administered 1 of 2 treatment combination schedules. In Arm 1, pts received ibr 560 mg once daily until progressive disease (PD) or unacceptable toxicity, combined with RTX 375 mg/m2 IV once weekly for 4 doses for the first 4 weeks of the study. In Arm 2, pts received a lead-in of ibr 560 mg for 8 weeks, and then concurrently with RTX 375 mg/m2 IV once weekly for 4 doses, followed by continuous ibr 560 mg until PD or unacceptable toxicity. The purpose of the Arm 2 design was to identify biomarkers that may predict ibr sensitivity or resistance. The primary endpoint was investigator-assessed ORR (2007 IWG criteria). Secondary endpoints included duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety.
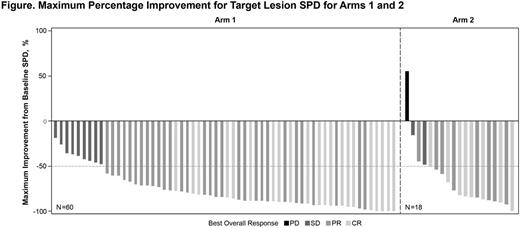
Results: Sixty pts were treated in Arm 1 and 20 in Arm 2. Median age was 58 and 55 years in Arm 1 and 2, respectively, ECOG PS1 in 22% and 40% of pts, stage III/IV disease in 80% and 95%, and grade 3a FL in 10% and 5%, respectively. All pts in Arm 1 and 85% in Arm 2 completed the 4-week RTX therapy. Median treatment duration was 20 mo in Arm 1 and 12 mo in Arm 2. At a median time on study of 22 mo for Arm 1, the ORR was 85%, with 35% (21/60) CRs and 50% (30/60) PRs. At a median time on study of 15 mo for Arm 2, the ORR was 75%, with 35% (7/20) CRs and 40% (8/20) PRs. Median time to best response was 2.7 mo for Arm 1, and 4.3 mo for Arm 2. Median DOR, PFS, and OS were not reached in either arm. The 12-mo PFS rates were 87% (Arm 1) and 77% (Arm 2), and the 12-mo OS rates were 98% (Arm 1) and 100% (Arm 2). The most common adverse events (AEs; ≥30%) included fatigue (67%), diarrhea (50%), nausea (47%), constipation (30%), and headache (30%) in Arm 1, and fatigue (75%), diarrhea (60%), nausea (55%), stomatitis (55%), dizziness and myalgia (45% each), paresthesia and maculopapular rash (35% each), and increased lacrimation and upper respiratory tract infection (30%) in Arm 2. Common grade ≥3 AEs in either Arm 1 or 2 (in >5% pts) included maculopapular rash (5% and 10%, respectively), fatigue (7% and 5%), pyrexia (3% and 10%), and diarrhea (Arm 2 only-10%). Serious AEs occurred in 20% of pts, with a higher frequency in Arm 2 vs Arm 1 (25% vs. 18%). Any-grade bleeding was reported in 33% of pts, with only 1 grade 2 bleeding event of petechiae in Arm 1, and 1 grade 3 event of rectal hemorrhage in Arm 2; all other bleeding events were grade 1. Any-grade atrial fibrillation occurred in 4 pts (all in Arm 1), leading to treatment discontinuation in 2 pts. Secondary malignancies were reported in 4 pts (3 in Arm 1 and 1 in Arm 2): Hodgkin's lymphoma (n=1; grade 3 and 5); fallopian tube cancer (n=1; grade 3); basal cell carcinoma (n=1; grade 2); and lung adenocarcinoma (n=1; grade 2). Overall, 40% of pts in Arm 1 discontinued ibr (progressive disease [PD]: 13%; AEs: 15%, pt decision: 7%; and investigator decision: 5%); 60% continued treatment. In Arm 2, 30% of pts discontinued ibr (PD: 20%; AEs: 10%); 70% of pts continued treatment.
Conclusions:In treatment-naïve pts with FL, ibr combined with 4 cycles of RTX continues to demonstrate robust clinical activity and durable responses, with a high ORR. In addition, the responses appear to improve over time with a higher CR rate observed with longer follow-up in this report (Arm 1). The study treatment was well tolerated, and no new/increased safety signals were observed with additional follow-up, with AEs being primarily grade 1-2 and as expected based on the experience with single-agent ibr and previously tested ibr and RTX combinations.
Fowler:Pharmacyclics, LLC, an AbbVie Company: Consultancy, Research Funding; Janssen: Consultancy, Research Funding. Nastoupil:AbbVie: Research Funding; Janssen: Other: Travel, Accommodations, Expenses, Research Funding; TG Therapeutics: Research Funding; Celgene: Honoraria. Knapp:Insys Therapeutics, Inc.: Consultancy, Other: Travel, Accommodations, Expenses; Pharmacyclics, LLC, an AbbVie Company: Consultancy, Other: Travel, Accommodations, Expenses, Research Funding. Flinn:Janssen: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; Gilead Sciences: Research Funding; ARIAD: Research Funding; RainTree Oncology Services: Equity Ownership. Chen:Seattle Genetics: Consultancy, Honoraria, Research Funding, Speakers Bureau; Millenium: Consultancy, Research Funding, Speakers Bureau; Genentech: Consultancy, Speakers Bureau; Merck: Consultancy, Research Funding. Bhatia:CHOP, LLC: Employment, Other: Leadership; Pfizer: Honoraria. Martin:Gilead: Consultancy, Other: travel, accommodations, expenses; Janssen: Consultancy, Honoraria, Other: travel, accommodations, expenses; Novartis: Consultancy; Teva: Research Funding; Celgene: Consultancy, Honoraria; Acerta: Consultancy. Suzuki:AbbVie: Equity Ownership; Pharmacyclics, LLC, an AbbVie Company: Employment, Other: Leadership; Travel, Accommodations, Expenses. Beaupre:AbbVie: Equity Ownership; Pharmacyclics, LLC, an AbbVie Company: Employment, Other: Leadership, Patents & Royalties, Research Funding. Neuenburg:AbbVie: Equity Ownership; Pharmacyclics, LLC, an AbbVie Company: Employment. Palomba:Juno: Consultancy; Janssen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal