Abstract
Background
Mantle cell lymphoma (MCL) is a biologically heterogeneous disease with a variable clinical course, ranging from indolent to highly aggressive. A subset of MCL patients with an indolent clinical course can be initially monitored and previous studies show that this strategy is not associated with inferior overall survival (Martin JCO 2009, Cohen Cancer 2016). However, uniform clinical or biologic criteria do not exist to identify appropriate candidates for observation. Prior studies have described features of indolent MCL including clinical presentation with leukemic phase disease and minimal lymphadenopathy and biological characteristics including low proliferative index, lack of expression of SOX-11 transcription factor, genetic stability without TP53 alterations, and IGHV somatic hypermutation. The aim of this study was to describe the clinical and biological characteristics of MCL patients who were initially observed (OBS) versus immediately treated (TX) and the associated outcomes.
Methods
Patients with histologically confirmed MCL diagnosed between 2000-2014 who were initially managed and subsequently followed at Memorial Sloan Kettering Cancer Center were included. Patients were considered to have been observed if the treating oncologist documented an intent to expectantly monitor the patient as an initial management strategy. There were no predefined criteria for observation versus immediate treatment. Overall survival was defined as date of tumor diagnosis to death from any cause.
Results
404 patients met the study inclusion criteria. 91 were initially observed and 313 were immediately treated. Treating oncologists documented different reasons for observation including: asymptomatic patient, lack of GELF criteria / low tumor burden, favorable biologic features (low Ki67, SOX-11 negative), leukemic phase disease without lymphadenopathy, or GI tract only disease. Patients in the OBS group were significantly more likely to have classic MCL morphology (versus blastic/blastoid morphology), normal LDH, less than two extranodal sites, leukemic phase disease, lower SUVmax, low-risk or low-intermediate risk IPI, and lower Ki-67 (<30%). MIPI scores were not significantly different across the two groups.
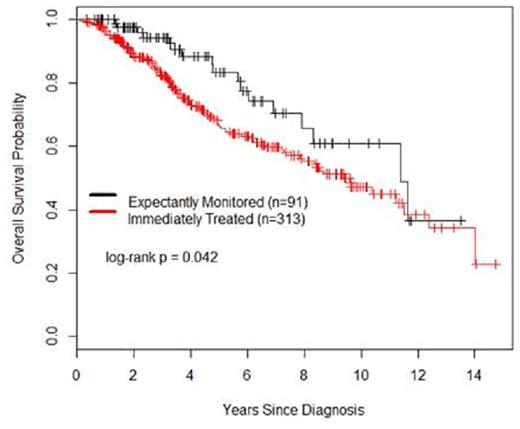
With a median follow up of 5 years for all patients, there was a superior overall survival (OS) for OBS patients versus TX patients, P=0.042, see Figure 1. The median OS for the OBS versus TX patients was 10.6 and 9.4 years, respectively. There was no difference in outcome between OBS and TX patients when comparing time from start of treatment to death, P=0.79. In univariate analyses, the factors associated with inferior OS were age>60, Karnofsky performance status (KPS) ≤70%, advanced stage disease, elevated LDH, bone marrow involvement, higher SUVmax, MIPI, IPI, Ki-67 ≥30%, and treatment decision at diagnosis (TX vs. OBS). In multivariate analysis of individual factors, only age>60 and KPS ≤70% were associated with inferior OS. In propensity score-adjusted analyses, OS was similar in both OBS and TX groups (hazard ratio 0.74, 95% confidence interval 0.43, 1.26, P=0.26).
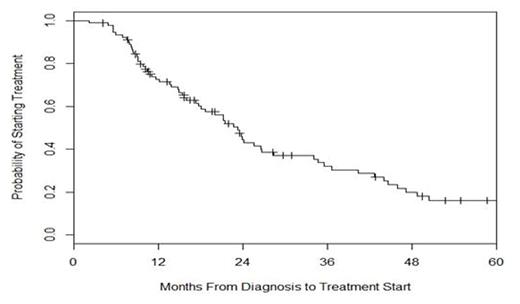
Among the 91 observed patients, 65 were started on therapy after a period of observation while 26 continue to be expectantly monitored. The median time from diagnosis to start of treatment was 23 months, see Figure 2. Of note, 25 patients have been monitored for 2-5 years and 5 patients have been monitored for ≥5 years. Using a Cox proportional hazards model to identify predictors of time from diagnosis to initiation of treatment in the OBS patients, early stage disease (P=0.037) and absence of lymphadenopathy (P=0.021) were significantly associated with a more prolonged period of observation.
Conclusions
Observation is an appropriate initial management strategy for a subset of newly diagnosed MCL patients and is not associated with a negative impact on overall survival. Due to limited availability of pathologic information, including Ki-67, SOX-11, and IGHV status, this report cannot correlate biologic features with outcome; however, this is the focus of ongoing studies. Based on our data, there are various clinical criteria that can be used to select appropriate candidates for observation, among them presence of early stage disease or non-nodal clinical presentation.
Kumar:Celgene: Research Funding; Seattle Genetics: Research Funding; Adaptive Biotechnologies: Research Funding; Celgene: Honoraria, Other: Scientific Advisory Board; Pharmacyclics: Research Funding. Hamlin:Novartis: Research Funding; Molecular Templates: Research Funding; Seattle Genetics: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Xencor: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Portola: Research Funding. Moskowitz:Celgene: Consultancy; Genentech: Consultancy; Merck: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Pharmacyclics: Research Funding. Zelenetz:Gilead Sciences: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal