Abstract
Background:
MCL is characterized by a dismal long term prognosis with a median overall survival of 3-5 years. Rituximab maintenance treatment (MR) improves survival of patients with follicular lymphoma, and its effect was assessed for MCL patients in several trials with inconsistent results. Other agents as bortezomib were also evaluated as maintenance therapy for MCL.
Aims:
We performed a systematic review and meta-analysis of RCTs in order to assess the effect of maintenance therapy on clinical outcomes of patients with MCL.
Methods:
We included RCTs that compared any type of maintenance to no maintenance or a different maintenance for patients with MCL, either in first line or relapsed disease. In July 2016 we searched The CochraneLibrary, MEDLINE, conference proceedings, and databases of ongoing trials. Two reviewers independently assessed the qualityof the trials and extracted data. The primary outcome wasall cause mortality. Secondary outcomes included progression free survival (PFS) and infectious adverse events. Relative risk (RR) for dichotomous data and hazard ratio (HR) for time to event datawere estimated and pooled using random-effects model.
Results
We identified 6 trials that reported relevant outcomes, conducted between the years 1998 to 2012 and randomizing 857 patients with MCL. Most patients were males, with a median age ranging from 57 to 70 years, and predominantly good performance status. Ninety-six percent of the patients received their first line of treatment. MIPI score was reported in three trials: 20 to 42 percent of patients had a high MIPI score. Induction therapy included rituximab in all trials. In five trials chemotherapy induction was applied and consisted of fludarabine, cyclophosphamide (FC) and mitoxantrone (Forstpointner, Blood 2006), cyclophosphamide, vincristine, adriamycin, prednisone (CHOP) or FC in one trial (Kluin-Nelemans et al.), bendamustine (Rummel et al.), DHAP followed by autologous stem cell transplantation (ASCT) (Le Gouill et al.), and CHOP/cytarabine followed by ASCT (Doorduijn et al.); in one trial rituximab was given alone (Ghielmini et al.). Maintenance consisted of rituximab in five trials and bortezomib in one trial (Doorduijn et al.). The control group received no maintenance in two trials and interferon alfa in one trial. All included trials were judged at low risk of selection bias, none were blinded.
No statistically significant effect on mortality rate was shown with rituximab maintenance therapy compared to no maintenance or interferon alfa RR 0.72, 95% CI 0.50 to 1.04, I2 of heterogeneity 57%, 751 patients. The RR of mortality with bortezomib maintenance was 0.67, 95% CI 0.12 to 3.71, but that is based on only 60 patients.
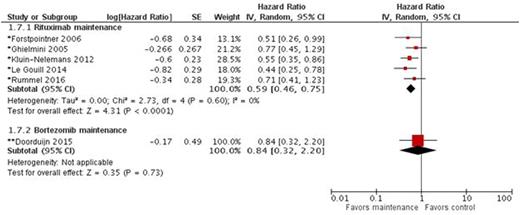
PFS improved with rituximab maintenance compared to no maintenance or interferon alfa: HR 0.59, 95% CI 0.46 to 0.75. With bortezomib maintenance vs. no maintenance the HR of event free survival was 0.84, 95% CI 0.32 to 2.20.
There was no statistically significant difference in infection rate with or without maintenance (RR 0.80, 95% CI 0.37 to 1.69, 419 patients).
Conclusions
Maintenance therapy improved PFS of patients with MCL, but no survival benefit could be shown. The pooled analysis is based mainly on the results of rituximab maintenance as data of the effect of bortezomib maintenance is scarce. The absence of significant increase of infection rate as opposed to maintenance rituximab in follicular lymphoma might be attributed to the small sample size. Based on these results patients treated for both first line and relapsed/refractory MCL should receive rituximab maintenance after achieving response to induction.
disease control (*progression free survival, **event free survival) of patients with MCL who responded to induction and treated with rituximab maintenance compared to observation or interferon alfa.
disease control (*progression free survival, **event free survival) of patients with MCL who responded to induction and treated with rituximab maintenance compared to observation or interferon alfa.
Dreyling:Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal