Abstract
Introduction: The combination of bendamustine (B) and rituximab (R) is an effective and relatively well tolerated treatment for B-cell malignancies. However, there is increased concern regarding infectious complications since FDA approval, in particular due to reports of prolonged and profound lymphopenia associated with BR. [Saito, Blood Cancer J 2015; Garcia-Munoz, Ann Hematol 2014] There have been numerous case reports of opportunistic infection (OI) with BR, such as viral reactivation (HBV, VZV, HSV, CMV, EBV) and Pneumocystis jiroveci. [Abkur, Clinical Case Reports 2015;Carter, Leuk Res 2011; Tsutsumi, Int J Hematol 2012; Lim, Ann Hematol 2011] A retrospective analysis conducted in Israel showed that B ± R therapy was associated with 47% incidence of infectious complications (ICs) with 2 out of 183 pts received antimicrobial prophylaxis (ppx) and 65% G-CSF use.[Gafter-Gvili, Blood 2014, abs 3077]Another study concluded that a prolonged period of VZV ppx may be advisable with BR.[Allen, Blood 2015, abs 4167] Brugger et al published a practice guide for B-based therapy with a section devoted to discussing potential ICs and considerations for antimicrobial ppx.[Brugger, Oncologist 2013] In prospective trials (StiL and Bright), Rummel et al reported a 37% incidence of unspecified infectious episodes and Flinn et al reported 55% incidence of all infections with 10% OI despite 30% G-CSF use.[Rummel, Lancet 2013, Flinn, Blood 2014] Antimicrobial ppx and G-CSF use were not mandated in those trials. With these reports and OI episodes in a few of our patients, we performed a retrospective analysis at MSKCC to evaluate the incidence of ICs and potential risk factors in patients treated with B and anti-CD20 antibody ± R maintenance.
Methods: Pts ≥18 year old with CD20+ NHL and received ≥2 cycles of B and anti-CD20 antibody (rituximab or ofatumumab) ± R maintenance from 2008 through 2015 were included. Pts were excluded if they received B monotherapy, switched treatment before completion of planned course, or underwent stem cell transplantation right after completion of bendamustine combination. Infection data were collected for up to 1 yr post B-based treatment with a cutoff date of 5/1/2016. Adverse drug events (ADEs) including neutropenia, neutropenic fever (NF), lymphopenia, time to lymphocyte recovery, and liver function abnormalities were graded according to CTCAE v4.0. Univariate analysis with Fisher's exact test was used to evaluate the potential risk factors (degree of lymphopenia and neutropenia, R maintenance, and line of therapy) for ICs.
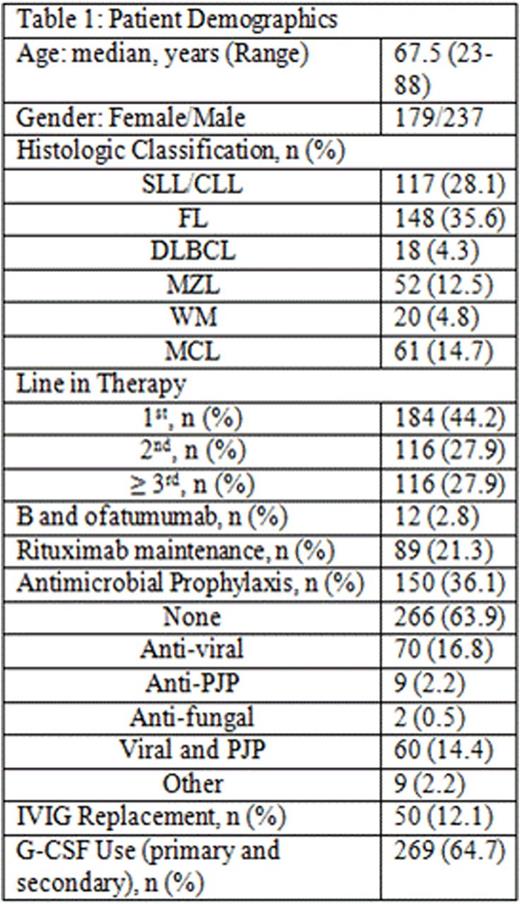
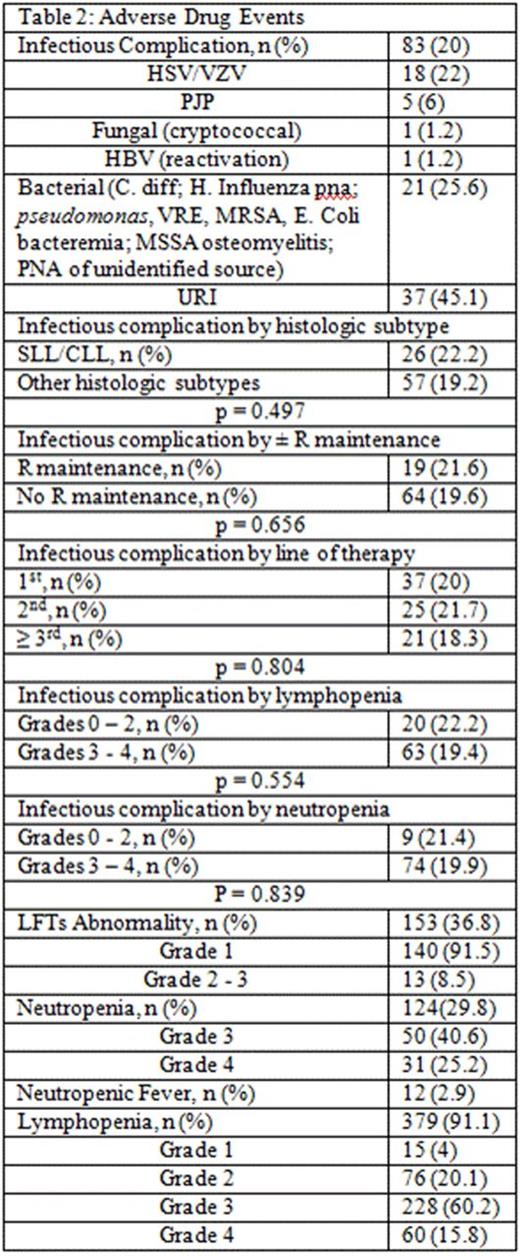
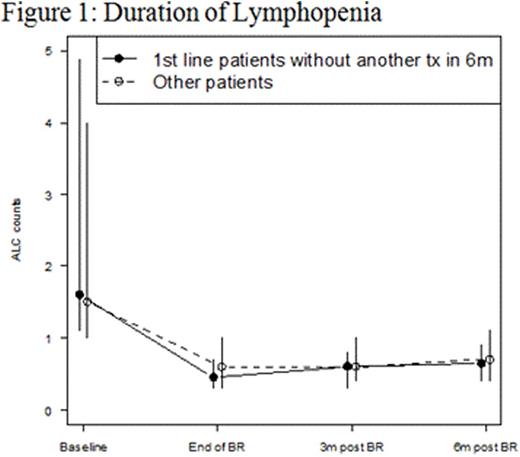
Results: 416 pts were included in this retrospective analysis (Table 1). Initial bendamustine dose ranged from 50mg/m2 to 120mg/m2, with 11.5% of pts requiring dose attenuation. 55.8% received B and anti CD-20 antibody as ≥ 2nd line therapy. The incidence of ICs was 20% (n = 83; 95% CI: 16 to 24%) and 6% (n = 25; 95% CI 3.7-8.5%) of which was OI in this cohort (Table 2). The 25 OI cases consisted of viral (n = 19), fungal (n = 1), and PJP (n = 5). Nine cases occurred during B-based treatment and 16 cases occurred up to a year post (one was on R maintenance). All 25 cases were associated with either no ppx (n = 21), early ppx cessation (≤ 1 month post) (n = 2), or non-compliance (n = 2). One pt died of disseminated histoplasmosis 1.5 years after completed rituximab maintenance without additional treatment. Antimicrobial ppx, mainly anti-viral and anti-PJP, was employed in 36.1% of pts and primary or secondary G-CSF ppx in 64.7%. ICs were not associated with SLL/CLL histology (p = 0.471), R maintenance (p = 0.843), line of therapy (p = 0.804), and grade of lymphopenia (p = 0.554) or grade of neutropenia (p = 0.839) (Table 2). However, OI was associated with lack of antimicrobial ppx (p = 0.048). Other ADEs included grade 3/4 neutropenia (65.8%), NF (2.9%), grade 3/4 lymphopenia (76%), and elevated liver function tests (91.5% grade 1). The median absolute lymphocyte counts nadired after cycle 3 and persisted for at least 6 months following completion of bendamustine combination (Figure 1).
Conclusions: The 20% incidence of infectious complication and 6% of opportunistic infection with bendamustine and anti-CD20 antibody combination at MSKCC are somewhat lower than that reported in prospective trials and retrospective analysis by Gafter-Gvili et al, possibly due to antimicrobial ppx and G-CSF use. We have implemented a prophylaxis guideline at MSKCC.
Hamlin:Celgene: Membership on an entity's Board of Directors or advisory committees; Xencor: Membership on an entity's Board of Directors or advisory committees; Molecular Templates: Research Funding; Seattle Genetics: Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees; Portola: Research Funding; Novartis: Research Funding. Kumar:Celgene: Honoraria, Other: Scientific Advisory Board; Celgene: Research Funding; Adaptive Biotechnologies: Research Funding; Seattle Genetics: Research Funding; Pharmacyclics: Research Funding. Moskowitz:Seattle Genetics: Honoraria, Research Funding; Merck: Honoraria; Bristol Myers Squibb: Honoraria. Moskowitz:Merck: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Noy:Pharmacyclics, LLC, an AbbVie Company: Other: travel, accommodations, expenses, Research Funding. Zelenetz:Gilead Sciences: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal