Abstract
Introduction:
Despite improvements with combination chemotherapy and/or radiation, up to one third of patients with classical Hodgkin lymphoma (cHL) relapse following primary therapy. The standard of care for these patients is salvage chemotherapy followed by autologous stem cell transplantation; however, this carries a high risk of treatment-related morbidity and is curative in only about one-half of patients. Histopathologically, HL is characterized by rare tumor-initiating Reed-Sternberg (RS) cells surrounded by a dense but functionally incapacitated inflammatory infiltrate. This immunosuppression is thought to be mediated by multiple mechanisms, including genetic amplification of PD-L1 expression on RS cells, loss of MHC class I and class II expression, and infiltration of regulatory T cells. Whether any components of the immune microenvironment are associated with disease recurrence or long-term outcomes in HL remains unknown.
Methods:
We identified 76 patients with newly diagnosed and 50 patients with relapsed cHL from 2000 to 2014 who had formalin-fixed, paraffin-embedded tissue available for immunohistochemical (IHC) analysis. IHC staining was performed as per standard protocols against B2M, MHC-I, MHC-II, FoxP3, and PD-L1. Scoring for B2M, MHC-I, and MHC-II was reported in a binary fashion. FoxP3 expression was scored as low (<12.5 cells/HPF), medium (12-50 cells/HPF), and high (>50 cells/HPF). PD-L1 expression was scored both within Reed-Sternberg (RS) cells and within the tumor microenvironment (TME) as low (<20% of cells) or high (20% of cells or higher). Differences in expression of B2M, MHC-I, MHC-II, FoxP3, and PD-L1 between the newly diagnosed and relapsed cohorts were analyzed by Fisher's exact and Chi-Square tests. The Kaplan-Meier method and Cox proportional hazards model were used to evaluate the relationship between clinical factors or IHC expression and progression-free survival (PFS) or overall survival (OS).
Patient Characteristics:
Samples from a total of 126 patients were identified. The median age was 34 (range 13-75); 25% were 45 or older. 43% of patients were female while 57% were male. With respect to histology, 70% of patients had nodular sclerosing, 15% had mixed cellularity, and 8% had other subtypes. 54% of patients had early stage disease while 46% had advanced stage disease. Of the early stage patients, 43% had favorable disease while 57% had unfavorable disease. B symptoms and bulky disease (>10 cm) were present in 30% and 25% of patients, respectively.
Outcomes:
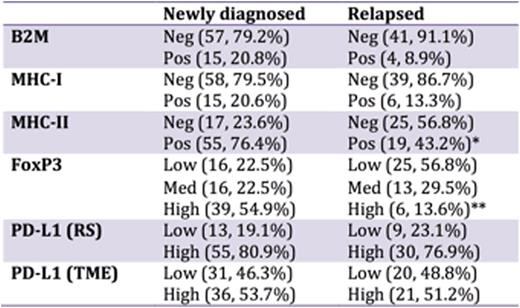
There was no significant difference in expression of B2M, MHC-I, or PD-L1 between newly diagnosed and relapsed patients. However, MHC-II loss was significantly more frequent amongst relapsed patients (56.8% versus 23.6% in newly diagnosed patients, p < 0.001) and high FoxP3 expression was significantly less frequent amongst relapsed patients (13.6% versus 54.9%, p < 0.001). Within the newly diagnosed cohort, the 10-year PFS and OS were 93.3% and 94.3%, respectively. No patterns of IHC expression were associated with outcomes in this cohort. Within the relapsed cohort, the 10-year PFS and OS were 53.5% and 66.9%, respectively. Univariate analysis identified high PD-L1 expression as independently associated with inferior PFS and OS in relapsed patients (p = 0.002 and 0.01 for PFS and OS respectively)
Conclusion:
Loss of MHC-II expression on RS cells and low FoxP3 expression in the TME are more common in relapsed as compared to newly diagnosed HL. Amongst relapsed patients, high expression of PD-L1 on both RS cells and the tumor microenvironment is associated with inferior progression-free and overall survival compared to low expression of PD-L1. Patients with high PD-L1 expression, in particular those with expression on both RS cells and the tumor microenvironment, might therefore benefit from early addition of checkpoint blockade as part of salvage therapy. Associations between expression of B2M, MHC-I, MHC-II, PD-L1, and FoxP3 and outcomes in patients treated with checkpoint blockade are ongoing.
Expression of immune-related genes in newly diagnosed and relapsed HL.
*p < 0.001 vs. newly diagnosed
**p < 0.001 vs. newly diagnosed
Expression of immune-related genes in newly diagnosed and relapsed HL.
*p < 0.001 vs. newly diagnosed
**p < 0.001 vs. newly diagnosed
Expression of PD-L1 on both RS cells and in the tumor microenvironment at disease relapse is associated with inferior progression-free survival to second-line therapy.
***p = 0.002
Expression of PD-L1 on both RS cells and in the tumor microenvironment at disease relapse is associated with inferior progression-free survival to second-line therapy.
***p = 0.002
Matsuki:Nippon Shinyaku: Honoraria; Celgene: Honoraria; Bristol-Myers Squibb: Honoraria. Moskowitz:Bristol Myers Squibb: Honoraria; Seattle Genetics: Honoraria, Research Funding; Merck: Honoraria. Moskowitz:Merck: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Dogan:Seattle Genetics: Consultancy; Cancer Genetics: Membership on an entity's Board of Directors or advisory committees; Consulting Cancer Panel: Membership on an entity's Board of Directors or advisory committees; Peerview Institute: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal