Abstract
Background:
Outcomes for patients with relapsed B- acute lymphoblastic leukemia (B-ALL) remain suboptimal. We and others have identified the Mitogen activated protein kinase/ Extracellular signal regulated kinase (MAPK/ERK) pathway as a primary driver of drug resistance in pediatric B-ALL. Constitutive activation of ERK has been found to be an independent predictor of complete response and is associated with other poor prognostic factors in adults with B-ALL. Mutations that affect activation of the Ras/Raf/MEK/ERK pathway have been found in up to 40% of children with newly diagnosed and relapsed ALL and these mutations correlate with early relapse and poor overall survival. However, we have demonstrated MAPK activation in the absence of Ras pathway mutations, suggesting alternative mechanisms of activation.
Hypothesis:
We hypothesize that activation of the MAPK pathway drives drug resistance in pediatric B-ALL. Activation of this pathway at diagnosis may portend a high likelihood of relapse and inhibition of the MAPK pathway may restore chemosensitivity to conventional agents.
Methods:
For our initial pilot study, a total of 24 patient samples from initial diagnosis obtained from Children's Oncology Group cell bank and from NYU, were thawed and placed either in serum-free media or media containing Trametinib (MEK1/2 inhibitor) at 1µM for 4 hours. Samples were then stimulated with Phorbol 12-myristate 13-acetate (PMA), a specific activator of Protein Kinase C, which activates MEK via Ras. Samples were then fixed with 4% paraformaldehyde, permeabilized with 95% methanol, and stained for Caspase 3, CD10, phosphorylated ERK (p-ERK), and p-AKT. All samples were simultaneously processed on the BD LSRII HTS and analyzed on FlowJo software (Treestar, V.10). Viable leukemic blasts were identified as CD10 positive and Caspase 3 negative cells, which were gated on for further analysis. Median fluorescence intensity (MFI) for phosphorylated proteins was measured, expressed as a ratio to isotype controls and compared between samples. MAPK activation was defined by maximal p-ERK levels upon stimulation with PMA. Inhibition was quantified as percentage decrease in maximal p-ERK levels (upon PMA stimulation) after treatment with Trametinib. Targeted Sanger sequencing of commonly mutated exons of Ras pathway genes (NRAS, KRAS, FLT3 and PTPN11) was performed on 14 samples based on availability of DNA.
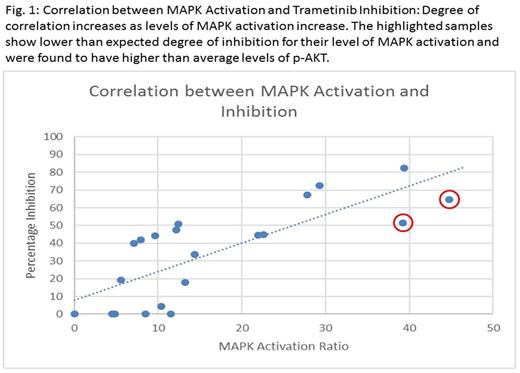
Results:
Of our 24 samples, 4 samples were excluded from analysis due to poor viability (<40% live cells), giving an assay success rate of 83.3%. We were able to successfully measure p-ERK in all 20 viable patient samples and also measure levels of baseline p-AKT in 16 samples. A wide range of p-ERK levels and MAPK activation was observed within patient samples (median activation ratio 12.3, range 4.4-44.7) and 7 samples showed markedly higher levels of activation with ratios between 21.9 - 44.78. Levels of inhibition by Trametinib ranged from 0-82.2%, with a median of 43%. Greater degree of MAPK activation was found to correlate strongly with higher levels of inhibition by Trametinib (overall correlation coefficient 0.72). In 2 samples, the levels of inhibition by Trametinib seemed lower than anticipated for levels of MAPK activation, but both of these samples showed higher than average levels of p-AKT (p-AKT to isotype ratio of 12.3-12.9, median of 2.4 in rest of the samples), suggesting a possible alternative mechanism of activation via the PI3K/AKT signaling pathway. DNA was obtained for Sanger sequencing in 14 of the 20 viable patient samples. Three of the 14 samples had point mutations in Ras pathway genes of which two had mutations in exon 2 of NRAS while one had a mutation in exon 14 of FLT3. Two of the seven patient samples with the highest MAPK activation had Ras mutations, which is consistent with our prior data showing that additional mechanisms of pathway activation are operative.
Conclusion:
We have successfully established a phospho-flow protocol that enables the measurement of MAPK activation in real time in patient samples. Based on our pilot data, we also conclude that MAPK activation in itself serves as a better predictive marker for susceptibility to MAPK inhibition, as opposed to Ras pathway mutation status alone. Ongoing investigation will allow correlation with outcome and the assay could serve in the future to identify those patients who might benefit from MEK inhibition or other nodes in the pathway.
Loh:Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Abbvie: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal