Abstract
Acute undifferentiated leukemia (AUL) and mixed phenotype acute leukemia (MPAL) are acute leukemias of ambiguous lineage representing less than 5% of all adult leukemias. Ontogenetically, they are thought to be caused by precursor lesions in early pluripotent hematopoietic progenitor cells having the ability to differentiate into both lymphoid and myeloid lineages. In contrast, common lymphoid and myeloid leukemias are likely derived from precursor lesions in committed progenitor cells. The molecular characteristics of the cell of origin of these leukemias have not been fully characterized.
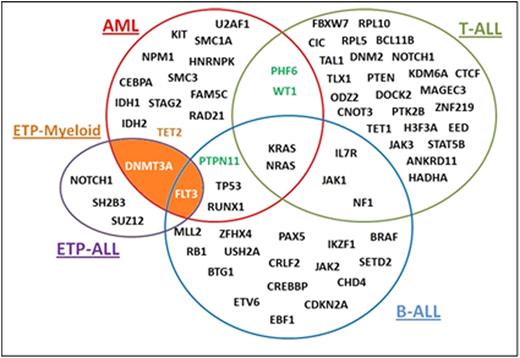
Given the ambiguous classification of these disorders, we used NGS to evaluate the molecular profiles of this unique subset of leukemias. Our panel tested for the 60 most commonly mutated genes in myeloid malignancies (MM), which can be used to further elucidate driver mutations that play a role in the pathogenesis of this unique entity. Molecular data was available for 18 of 35 patients (pts) (51%), of whom 8 were T/myeloid, 7 were B/myeloid, and 3 were AUL. 14 pts out of the 18 had samples at the time of initial diagnosis, 3 AUL cases harbored mutations commonly seen in MM, including one patient with DNMT3A and a germ line JAK3 mutation, another with U2AF1 and PHF6 mutations, and one pt with SF3B1 and MECOM mutations. Of the 5 B/myeloid cases, 4 had only one mutation detected by the sequencing panel, including TET2, PTPN11, KDM6A, and PHF6, and one had no mutations, but did have complex cytogenetics. Of interest, we identified four patients with T/myeloid MPAL that harbored a FLT3-ITD mutation. Notably, one of these cases had additional DNMT3A and TET2 mutations while another case had a WT1 mutation. We assert that the mutational data for MPAL supports that T-myeloid MPAL encompasses a borderland between early T precursor (ETP)-ALL/AML in addition to more typical cases of T-ALL/AML given its similar immunophenotype to ETP ALL (CD117, CD13 and CD33 positive). The overlap between these two entities is illustrated in the figure below. With this immunophenotyping and molecular profiling we propose a new provisional entity, ETP-myeloid MPAL in addition to already existing ETP ALL.
Of 1250 pts with acute leukemia diagnosed from 1997-2016 at Cleveland Clinic, 35 had immunophenotypic characteristics of ambiguous leukemia based on WHO criteria. The median age at diagnosis was 50 years, and 22 (63%) were male. There were 31 MPAL (16 T/myeloid, 15 B/myeloid) and 4 AUL cases. Among the 31 MPAL patients, 2 were mixed lineage chimera (subclones), and 29 had true biphenotypic features. 48% of the cohort had normal cytogenetics at diagnosis, 23% had other cytogenetic abnormalities, 14% had complex karyotype, 9% had MLL gene rearrangement, and 6% had t(9;22). 62% of pts were treated with acute lymphoblastic leukemia (ALL) directed induction chemotherapy (IC), while 38% were treated with acute myelogenous leukemia (AML) directed IC. Rates of complete remission (CR) were significantly higher in pats treated with ALL-IC regimen (82%) versus AML-IC regimen (23%) (p=.003). In our study, two cases with the recurrent lymphoid mutations WT1 and KDM6A, that achieved CR with upfront ALL-IC, while one case with FLT3-ITD, DNMT3A, and TET2 mutations achieved CR with AML-IC treatment. Pts <40 yrs of age had significantly better median OS (41 months) than patients over 40 yrs old (16 months) (p=0.03). MPAL pts with normal cytogenetics had worse median OS (16 months) compared to any other cytogenetic group (25 months) (p=.05).
In conclusion, clinical evaluations of ambiguous leukemias demonstrate that those with normal karyotype have the worst outcome of all pts diagnosed with MPAL. This can be partly explained by mutations that are detected at the time of diagnosis such as FLT3-ITD and DNMT3A, which have been associated with poor survival in AML with normal karyotype. Identification of actionable targets like FLT3-ITD can improve clinical outcomes for this subset of patients prior to BMT or for those ineligible for BMT. The existence of a unique subset of pts with immunophenotypic overlap between ETP-ALL/AML, that were classified in routine practice as T/myeloid leukemia, are important to recognize due to their unique actionable phenotype. Molecular profiling of these cases identified gene mutations more akin to MM. We propose that sequencing may play an important role in optimizing the therapeutic approach for patients with ambiguous lineage leukemia.
Carraway:Novartis: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Baxalta: Speakers Bureau; Celgene: Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal