Abstract
Background: The colony stimulating factor 3 receptor(CSF3R) plays an important role in granulocyte differentiation and acquired mutations have been described in different myeloid malignancies. Besides chronic neutrophilic leukemia (CNL) and severe congenital neutropenia (SCN), mutations in CSF3R (CSF3Rmut) have also been reported in acute myeloid leukemia (AML) at low frequency and recently have been associated with CEBPA mutations (Lavallèe et al., Blood 2016; Maxson et al., Blood 2016) in adult and pediatric AML. First studies suggest sensitivity of CSF3Rmut CNL against JAK kinase inhibitors suggesting the possibility for targeted therapy for a subset of CSF3Rmut AML patients.
Aims: The evaluation of CSF3Rmut in AML with intermediate-risk karyotype for frequency and association with other mutations with special focus on CEBPA mutation status.
Methods: We analyzed 274 cases with intermediate risk de novo AML for CSF3Rmut by next generation sequencing (Illumina, San Diego, CA) of exon 14 and 17 (sensitivity: 1%), which contain the mutational hotspot regions of CSF3R. As CSF3R mutations were associated with CEBPA mutations in the above-mentioned studies, we analyzed a selected cohort of 179 CEBPA mutated cases in comparison to 95 cases with CEBPA wild type.202 cases (74%) had normal karyotype (CN-AML) and 72 (26%) had intermediate-risk aberrant cytogenetics according to MRC criteria. Female/male ratio was 127/147 and age ranged from 16- 88 y (median: 64 y). Mutation status of the following genes were available in all cases: ASXL1, CEBPA, FLT3-ITD, FLT3-TKD, GATA2, IDH1/2, KRAS, KMT2A-PTD, NPM1, NRAS, RUNX1 and WT1. All mutations with a variant allele frequency (VAF) < 10% were defined as subclonal. 95 cases had wild type CEBPA (CEBPAwt), 92 cases were CEBPA single mutated (CEBPAsm), 81 cases were CEBPA double mutated (CEBPAdm), 6 cases showed CEBPA mutations withloss of heterozygosity (CEBPA LOH).
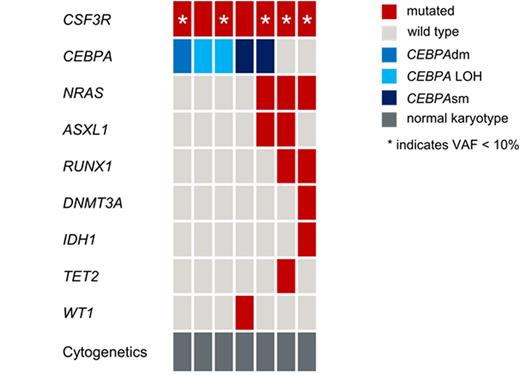
Results: Overall, in 7/274 patients (3%) CSF3Rmut were detected. This is slightly higher than expected in an unselected AML cohort (Sano et al., Br J Haematol 2015). Cytomorphology revealed AML M0 (n=1), AML M1 (n=2), AML M2 (n=3) and AML M4 (n=1). With regard to immunophenotype CSF3Rmut cases did not differ from CSF3Rwt cases which may be due to limited case numbers. All CSF3Rmut cases had CN-AML. The activating transmembrane mutation p.Thr618Ile, which has been identified as common mutation in CNL was the most frequent mutation (4/7). In addition two mutations leading to a truncated receptor (p.Ser715* and p.Gln749*) and a previously not described frame-shift mutation leading to a defecting splice variant (p.Ser783Glnfs*6) were identified. The median CSF3R VAF was 7% (range 2-45%). In 5/7 cases, CSF3Rmut was subclonal (VAF <10%, see figure). CSF3Rmut cases showed a median of 2 concomitant mutations (range 1-4). The most frequent concomitantly mutated gene was CEBPA (5/7 cases; 2 cases CEBPAsm, 1 case CEBPAdm and 2 cases with CEBPA LOH), followed by NRAS (3/7), ASXL1 (2/7) and RUNX1 (2/7). IDH1, TET2 and WT1 were mutated in one case each (see figure). Mutations in NPM1, FLT3, KMT2A or GATA2 were never detected. Interestingly, in CEBPAdm and CEBPA LOH cases, CSF3R was the only concomitantly mutated gene, whereas CEBPAsm cases harbored mutations in at least 1 gene in addition to CSF3R. In 2/7 CSF3Rmut cases we analyzed changes in the patterns of genetic lesions between diagnosis and relapse. Case 1 harbored CEBPAdm and a subclonal CSF3Rmut (VAF: 7%) at diagnosis. At relapse the CEBPAdm persisted, whereas CSF3Rmut was lost. Case 2 harbored two germline CEBPAmut and one somatic CEBPAmut at diagnosis. In addition, mutations in CSF3R and WT1 were identified. At relapse both the somatic CEBPAmut and WT1mut persisted, whereas CSF3Rmut was lost. These results indicate again that the CSF3Rmut was present only in a subclone of CEBPAmut AML in both cases.
Due to the limited number of CSF3Rmut cases, we did not perform survival analysis in this study.
Summary: 1) CSF3R mutations are rare and predominantly subclonal events in AML and therefore may not be a suitable target for directed therapy.2) CSF3Rmut prevalently occur with CEBPA mutations but are not limited to CEBPAdm.
Fasan:MLL Munich Leukemia Laboratory: Employment. Haferlach:MLL Munich Leukemia Laboratory: Employment, Equity Ownership. Perglerová:MLL2 s.r.o: Employment. Kern:MLL Munich Leukemia Laboratory: Employment, Equity Ownership. Haferlach:MLL Munich Leukemia Laboratory: Other: Part Owner MLL Munich Leukemia Laboratory.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal