Abstract
Introduction: Exportin-1 (XPO1), a nuclear transport protein critical for the export of tumor suppressor proteins (TSPs) and select mRNAs to the cytoplasm, is highly expressed in acute myeloid leukemia (AML) and correlates with poor survival. Selinexor, an oral, first-in-class, selective inhibitor of nuclear export, blocks XPO1 function. We previously reported that sequential treatment of AML blasts using the hypomethylating agent decitabine followed by selinexor exhibited strong anti-leukemic effects in vivo by inducing the expression of silenced TSPs that are kept in the nucleus by XPO1 inhibition (Ranganathan, Blood 2015).
Methods: Based on these findings, a phase I dose-escalation study was initiated to evaluate the safety, feasibility, maximum tolerated dose (MTD), recommended phase 2 dose (RP2D), and preliminary clinical activity of selinexor in combination with decitabine in poor-risk AML pts (NCT02093403). Adults with relapsed or refractory (R/R) AML and older (age ≥60) unfit pts with untreated AML were eligible. Pts received 10-day decitabine induction(s) at 20mg/m2 on days 1-10 for up to four 28-day cycles in combination with selinexor once daily, twice weekly beginning on day 11. Pts with <5% marrow blasts after any cycle received 5-day decitabine maintenance with selinexor as described.
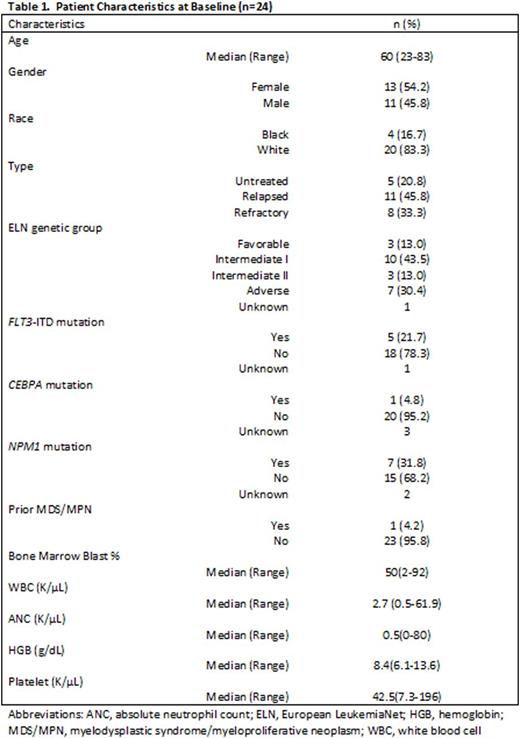
Results: Twenty-four pts were enrolled (see Table 1 for baseline pt characteristics). Nineteen pts had R/R AML (median of 3 prior chemotherapy regimens, range 1-4); five pts had previously untreated AML. Seventeen pts were treated on the dose escalation phase receiving selinexor doses ranging from 23mg/m2 to 55mg/m2. After two patients in DL 1 declined continuation of study therapy beyond cycle 1 due to grade <3 GI toxicities (nausea, anorexia), dosing of selinexor was limited to days 11, 13, 18, and 20 of each cycle (twice weekly for only 2 weeks instead of 3 weeks). At the maximum administered dose of 55mg/m2, three pts were treated with no grade ≥3 drug-related toxicities, but two pts declined continuation of study therapy after cycle 1, due to chronic low-level GI toxicities. Based on this and also emerging experience from a separate trial with single-agent selinexor, dosing of selinexor was changed to a flat dose of 60mg (~35 mg/m2) given twice weekly. Six additional pts were enrolled at 60mg of selinexor with improved tolerability; this is the RP2D. Overall, the most common grade ≥3 non-hematologic toxicities regardless of attribution were asymptomatic hyponatremia (n=17, 71%), febrile neutropenia (n=11, 46%), sepsis (n=10, 42%), hypertension (n=9, 38%), hyperglycemia (n=8, 33%), hypophosphatemia (n=7, 29%) and lung infections (n=7, 29%). Notably, the median number of treatment cycles given was only one (range 1-5). Ten pts discontinued study participation due to intolerable regimen related toxicities (nausea, fatigue, anorexia, and weight loss), five of these after cycle 1. Other reasons for treatment discontinuation included refractory AML (n=6), induction death during cycle 1 (n=3), death in remission (unknown cause, n=1), allogeneic transplantation (n=3), and uncontrolled infection (n=1). Of the five previously untreated pts, two achieved complete response (CR), and 2 had CR with incomplete count recovery (CRi). Of these responders, pts presented with normal karyotype (n=2), monosomy 7 (n=1), and del(7q), +13 (n=1). The latter two pts achieved cytogenetic remission. Of the 19 R/R pts, three had induction death not felt to be drug related. Of the 16 other pts in the R/R group, one patient achieved CR, two had CRi, one had marrow CR (mCR). The CR/CRi/mCR rate for the entire cohort was 33%. For untreated pts, the CR/CRi/mCR rate was 80% and, for R/R pts the CR/CRi/mCR rate was 21%. Six of the R/R pts subsequently underwent allogeneic stem cell transplant (four with no evidence of AML at the time of transplant).
Conclusions: The combination of selinexor plus decitabine is an active regimen in poor-risk AML pts, with CR/CRi/mCR rate of 80% in older untreated and 21% in R/R AML pts. Modification of selinexor exposure (flat dose of 60mg given twice weekly for 2 weeks after decitabine) improved tolerability of the regimen. Correlative studies, including targeted mutation analysis to assess whether specific mutations are associated with response, are ongoing and will be presented.
Bhatnagar:Karyopharm: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal