Abstract
Introduction
Tyrosine kinase inhibitors (TKIs) such asimatiniband dasatinib have markedly improved treatment results in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL). Almost all patients achieve at least a complete hematological remission with induction therapy consisting of TKImonotherapyor TKI in combination with reduced-intensity chemotherapy and corticosteroids. In eligible patients, allogeneic hematopoietic stem cell transplantation (alloHSCT) has been recommended in first complete remission, but its role inpatientsachieving rapid and deep molecular remissions with TKI-driven therapy is unresolved.
Methods
We analyzed data fromPh+ ALL patients in the Finnish Hematological Registry (FHR), a population-based database maintained by the Finnish Hematology Association, which includes patients participating in clinical study protocols sponsored by theTheFinnish Leukemia Group, and patients treated outside of clinical trials. The FHR contains detailed information on baseline (demographics, laboratory values,cytogenetics, molecular assays) and follow-up (therapies given and outcome). Minimal residual disease (MRD) was evaluated by standardized RQ-PCR for the bone marrow BCR-ABL1 transcripts with a minimum sensitivity of 10e-5. Therapy responses were coded according to the EuropeanLeukemiaNetguidelines.
Data from 128Ph+ALLpatients diagnosed between 1983-2016 were included in the analyses. Survival outcomes were calculated with the Kaplan-Meier method and compared with the log-rank test. Differences between groups were evaluated with the independent samples t-test for parametric numeric variables.
Results
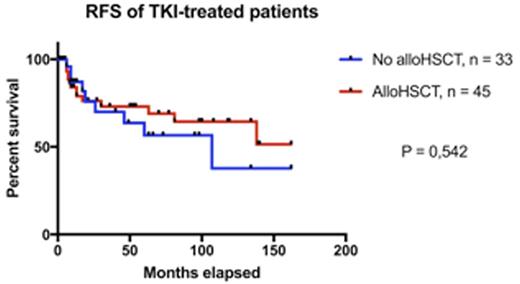
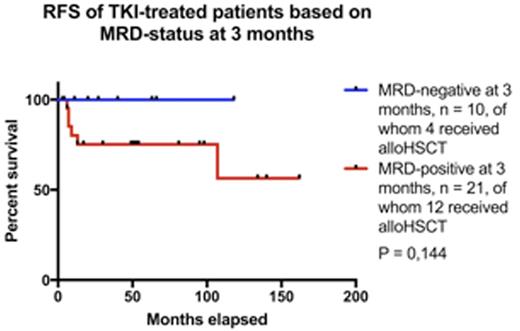
Of the 128 patients included in the analyses, 78 patients (61%) had received TKI treatment and 50 patients were treated prior the TKI era. The TKIs used wereimatiniband dasatinib and majority of patients concurrently received combination chemotherapy for induction and consolidation. Of the patients not treated with TKIs, 19/50 (38%) received an allotransplant and the overall survival (OS) at 5 years was 58% in the allotransplanted vs. 3% in thenontransplantedpatients (P<0.001). Of the patients treated with TKIs, 45/78 (58%) patients received analloHSCT. The mean age in thealloHSCTgroup was 41 years and in the non-alloHSCTgroup 62 years. OS at 5 years was better in thealloHSCTgroup (62% vs. 48%, P=0.004), but when analyzing causes of death, more deaths due to causes other than leukemia or its treatment were observed in the non-alloHSCTpatients (21% vs. 0 %), related to the competing causes of death in this older group of patients. In addition, there was more treatment-related mortality inalloHSCTpatients (22% vs. 6%). Relapse-free survival did not differ between transplanted and non-transplanted patients at 5 years (73 % vs. 57 %, P=0.42; Figure top panel). In TKI-treated patients, a trend for better OS was observed in patients who were MRD-negative at 3 months (Figure bottom panel).
Discussion
Our data indicate that up to 50% of patients withPh+ALLexperience long-term survival with TKI-driven therapy and noalloHSCT. However, robust predictive biomarkers are needed for selecting patients in whom the treatment-related mortality and morbidity ofalloHSCTare not warranted and could be treated with TKI-driven therapies only. MRD-negativity at 3 months may select for better outcome, but larger studies are needed for confirmation. In addition, disease-specific genomic andtranscriptomicprofiles (e.g. IKZF1, CDKN2 mutations) andimmunoreconstitutionmay prove valuable in this context. The advent of novel potent MRD-eradicating agents, such asbispecificCD3/CD19 antibodies, may further indicate re-evaluation of the role ofalloHSCTinPh+ALL.
Mustjoki:Bristol-Myers Squibb: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Ariad: Research Funding. Säily:Celgene: Other: Educational grant for congress participation; Amgen: Other: Educational grant for congress participation. Remes:Teva: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Janssen-Cilag: Consultancy, Membership on an entity's Board of Directors or advisory committees. Porkka:Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal