Abstract
Introduction
Innate immune complement activation may contribute to sickle cell disease (SCD) pathogenesis. The alternative complement pathway is abnormally activated in SCD and is additionally activated by phosphatidylserine (PS) on the outer leaflet of SS-red blood cells (SS-RBC). PS on the surface of SS-RBC and activated platelets accelerates the assembly of prothrombinase complexes leading to generation of thrombin, which can cleave circulating C5 protein into two biologically active fragments, C5a and C5b. C5a is an anaphylatoxin, a potent pro-inflammatory mediator, that can activate leukocytes, platelets, and endothelial cells, all of which play a role in vaso-occlusion (VO). Active C5b fragments stimulate the formation of membrane attack complexes (MAC) on SS-RBC that increases their susceptibility to lysis. We hypothesize that complement activation on the surface of SS-RBC may stimulate VO and RBC turnover in SCD.
Methods and Results
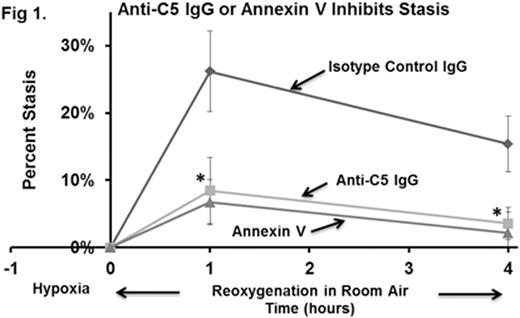
Whole blood from Townes-AA, -AS, and -SS mice (n=4) was collected in EDTA and RBC were immunostained with erythroid specific anti-Ter119 (Ly-76) IgG conjugated to PE-Cy7, anti-C5b-9 (MAC) IgG conjugated to Alexa Fluor 647, and anti-C3 (also specific for activation fragments C3b, iC3b, C3d and C3dg) IgG conjugated to PE. The percentages of Ter119-positive RBC that were positive for MAC were 6.3%, 6.5%, and 26.0% for AA-, AS-, and SS-RBCs, respectively (p<0.01 SS vs. AA and AS) suggesting enhanced C5 activation in Townes-SS mice. However, EDTA plasma C5a levels measured by ELISA were not significantly different between Townes-AA, -AS and -SS mice. C3 activation fragments were also found on a subset of MAC positive RBC. We used a mouse model of hypoxia/reoxygenation (H/R)-induced stasis to investigate the role of complement in VO. Townes-SS mice with implanted dorsal skin-fold chambers were infused with 30 µg of either anti-C5 IgG mAb BB5.1 (which blocks murine C5 cleavage ) (n=4), annexin V (n=2), or with isotype control IgG (n=4) 30 minutes prior to H/R (1 hour of hypoxia at 7% O2, followed by reoxygenation for 4 hours in room air). Percent microvascular stasis (% non-flowing venules) in the subcutaneous venules in the dorsal skin fold chamber window was measured using intravital microscopy at 1 and 4 hours post-hypoxia. Percent stasis was significantly lower in the Townes-SS mice receiving anti-C5 IgG or annexin V than the mice receiving the isotype control IgG at 1 and 4 hours post-hypoxia (Figure 1, means ± SD, *p<0.01 anti-C5 IgG or annexin V vs. control IgG). This finding implies that H/R may acutely activate C5 , thus leading to stasis. Initial studies suggest increased plasma C5a levels in Towne-SS mice treated with isotype control IgG compared to mice treated with anti-C5 IgG in response to H/R.
Conclusions
These results demonstrate an increased percentage of SS-RBC expressing MAC on their surface and inhibition of H/R-induced stasis with anti-C5 IgG. However, circulating C5a levels were not different between untreated Townes-AA, -AS, or -SS mice suggesting that C5a is not chronically elevated in SCD. Rather, the generation of C5a may occur locally and in the acute setting. Current studies underway are examining the effects of anti-C5 IgG on MAC deposition and RBC half-lives; the mechanism(s) of complement activation in SCD mice (classical, alternative, tic-over, etc); the ability of recombinant murine C5a to induce stasis; and complement activation markers on RBC and in plasma from SCD patients and controls.
Chen:Imara: Research Funding. Belcher:Imara: Research Funding; CSL-Behring: Research Funding. Vercellotti:Imara: Research Funding; CSL-Behring: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal