Abstract
Background:
Splanchnic vein thrombosis (SVT) includes extrahepatic portal vein obstruction (EHPVO), Budd-Chiari syndrome (BCS) and mesenteric vein thrombosis. Myeloproliferative neoplasms (MPN) account for the majority of non-cirrhotic SVT and are diagnosed in 30% of patients with EHPVO and 40% with BCS. Recurrent thrombosis is a common and significant complication in patients with PVT and MPN. Causes of thrombosis in this patient group are unknown; it is likely multifactorial, with proposed risk factors including increased platelet mass and platelet hyper-reactivity. There is evidence that in general, patients with MPN have increased platelet reactivity when compared to healthy controls, but, there is no data looking specifically at patients with SVT. However patients with SVT clearly demonstrate a significantly more aggressive phenotype in terms of systemic thrombosis, with risk of recurrent thrombotic episodes. MASCOT is a multicenter observational study assessing morbidity and portal circulation in patients with SVT, in a subset of whom we have assessed platelet activity.
Methods:
Whole blood flow cytometry was used to assess platelet activity by surface expression of P-selectin (CD62P) an established marker of platelet activity. We measured CD62P at baseline in unstimulated platelets and following stimulation with increasing concentrations of thrombin receptor activator protein (TRAP), range 10-50μmol. The guidelines from the European consensus on platelet flow cytometry were used for this assay (Schmitz G et al 1998). Demographic and clinical data were collected. Results were analysed in GraphPad Prism using t-tests; all values displayed as mean (95% confidence interval).
Results:
We assessed platelet activity in 14 patients with SVT using healthy controls (n=6) for comparison. In our patient cohort 8/14 (57%) were male and 6/14 (42%) female. The average age of our patients was 49 years. 12/14 (85%) were positive for the JAK2 mutation and 2/14 (15%) had a positive calreticulin mutation (CALR+). 2/14 (14%) patients had myelofibrosis (MF), 6/14 (42%) polycythaemia vera (PV), 4/14 (28%) essential thrombocytosis (ET) and 2/14 (14%) were positive for the JAK2 mutation but did not show evidence of MPN in their bone marrow and had normal blood counts. 10/14 (71%) were on warfarin alone, 2/14 (14%) on warfarin and aspirin, 1/14 (7%) on rivaroxaban and 1/14 (7%) on aspirin alone.
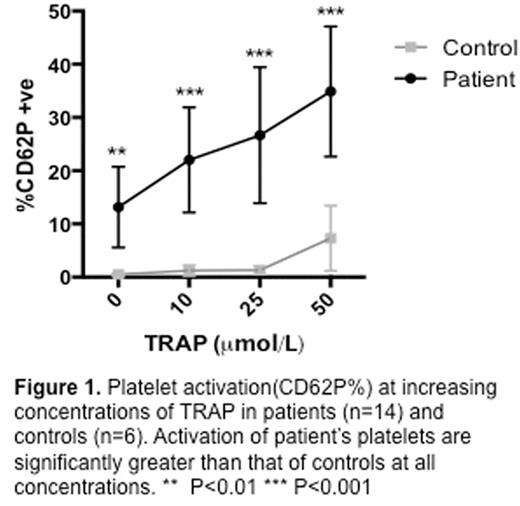
Platelet activity as measured by CD62P expression was increased in patients with SVT at baseline compared with controls 13.1% (5.5-20.7) vs 0.5% (0.2-0.7) (p=0.003). Platelet activation and reactivity was significantly greater in the patient group compared with controls at all concentrations of TRAP (figure 1). Control platelets were relatively unreactive to trap stimulation and a significant increase from baseline in CD62P at the highest concentration of TRAP used (50 μmol) 0.5% (0.2-0.7) vs 7.31% (1.17-13.4) (p=0.03), however this is a modest increase, the biological significance of which is uncertain.
In the patient population, the platelets were hyper-reactive to TRAP induced up-regulation of CD62P. This was significant when comparing unstimulated platelets at the 25 and 50 μmol concentrations 13.1% (5.5-20.7) vs 22.6% (13.8-39.4) (p=0.0008) and 13.1% (5.5-20.7) vs 34.9% (22.6-47.1) (p<0.0001) respectively. Although not significant at the lower concentration of 10 μmol TRAP there was a trend towards significance 13.1% (5.5-20.7) vs 22% (12.1-38.9) (p=0.07).
Discussion:
These results demonstrate that patients with SVT have both significantly increased baseline markers of platelet activation and increased reactivity to stimulation. As part of this longitudinal study, we propose to monitor changes in platelet activity in this patient cohort over time to assess whether or not changes in treatment modality influence platelet activity and whether or not changes in platelet activity can predict further thrombotic episodes; this data will be available in the coming year. Our data provides a platform for further investigation; in due course we will control this data using patients with MPN without thrombosis, as further patients within our study group are analysed.
Chen:Novartis: Other: Advisory Board. Sekhar:Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal